Collagen
Original Editor - Your name will be added here if you created the original content for this page.
Top Contributors - Lucinda hampton and Kim Jackson
Introduction[edit | edit source]
Collagen is principal protein of the skin, tendons, ligaments, cartilage, bone, and connective tissue. Depending upon the degree of mineralization, collagen tissues may be rigid (bone) or compliant (tendon) or have a gradient from rigid to compliant.
About one-quarter of all of the protein in our body is collagen. Collagen provides structure to our bodies, protecting and supporting the softer tissues and connecting them with the skeleton.[1]
- Type I fibrils are especially able to be stretched, gram-for-gram, being stronger than steel.[2].[3][4]
- Collagen production declines exposure to oxidative stressors eg smoking and UV light. production also declines with age, as is of a poorer quality. This impacts the appearance of skin, the maintenance of joint health, and much more.[5]
- Cosmetic lotions that claim to increase collagen levels are unlikely to do so, as collagen molecules are too large to be absorbed through the skin[2].
Types[edit | edit source]
There are close to 30 different types of collagen that have been identified so far. The most abundant type of collagen present in the human body is that of Type I (over 90% of the collagen in the body being type I[2]) with significant amounts of Type II,III and IV also accounted for.
- Collagen I- found in bones, tendons, organs.
- Collagen II- found mainly in cartilage
- Collagen III- found mainly in reticular fibres ( fine fibrous connective tissue occurring in networks to make up the supporting tissue of many organs).
- Collagen IV- found in the basement membrane of cell membranes (a thin noncellular layer located between epithelial cells and the connective tissue that underlies them, composed of collagen and other proteins and having a variety of functions including support and filtration)[6]
- Collagen V- found in hair, nails[4]
Collagen Synthesis[edit | edit source]
Synthesis takes place first inside the cell then outside the cell.
- The production of collagen starts with procollagen—the substance secreted by your cells. It goes through processing in the endoplasmic reticulum and Golgi body. This whole process needs vitamin C.
- Once outside the cell peptide chains are cleaved and tropocollagen is formed. These tropocollagen molecules gather to form collagen fibrils, via covalent cross-linking. Multiple collagen fibrils form into collagen fibers.
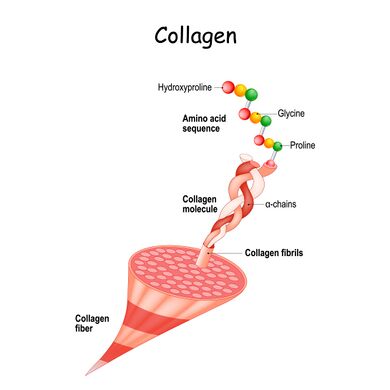
Structure[edit | edit source]
Each fiber of collagen contains thousands of individual collagen molecules that are bound together by cross-linking and staggered covalent bonds (the strongest bonds that exist between protein molecules).
The collagen molecules themselves are made from a triple helix—three polypeptides or strings of amino acids chains twisting around each other.
- There are 1,050 amino acids in each of the three chains that make up collagen. And they’re held together with hydrogens—the smallest atom.
- Glycine is amino acid that takes up the middle of the triple helix structure because it’s the only one that can fit. Glycine is an amino acid that has a single hydrogen atom as its side chain. It is the simplest amino acid.
- These long fibers don’t just exist as single protein ropes. Collagen can come together to form striated horizontal sheets.[5]
Collagen-Related Disorders[edit | edit source]
There are many types of disorders associated with collagen. Collagen-related diseases most commonly arise from genetic defects (one thousand mutations have been identified in twelve out of more than twenty types of collagen) or nutritional deficiencies that affect the biosynthesis, assembly, posttranslational modification, secretion, or other processes involved in normal collagen production. These include:
- Osteogenesis imperfecta (Brittle Bone disease)
- Chondrodysplasias eg achondroplasia
- Epidermolysis_bullosa_dystrophica
- Atopic Dermatitis
- Vitamin C Deficiency (Scurvy) (vitamin c is essential in collagen synthesis)[4]
References[edit | edit source]
- ↑ A level biology Collagen Available: https://alevelbiology.co.uk/notes/structure-of-collagen/(accessed 17.6.2022)
- ↑ 2.0 2.1 2.2 Medical news today What is collage and why do people use it. Available from:https://www.medicalnewstoday.com/articles/262881.php (last accessed 13.2.2020)
- ↑ Lodish H, Berk A, Zipursky SL, Matsudaira P, Baltimore D, Darnell J. Collagen: the fibrous proteins of the matrix. Molecular Cell Biology. 2000;4 Available from:.https://www.ncbi.nlm.nih.gov/books/NBK21582/ (last accessed 11.2.2020)
- ↑ 4.0 4.1 4.2 Proteopedia Collagen Available from:https://proteopedia.org/wiki/index.php/Collagen_Structure_%26_Function (last accessed 11.2.2020)
- ↑ 5.0 5.1 Ask the scientist Collagen Available from:https://askthescientists.com/collagen/ (last accessed 12.2.2020)
- ↑ Your dictionary Basement Membrane Available from:https://www.yourdictionary.com/basement-membrane (last accessed 12.2.2020)