Meningomyelocele
Introduction[edit | edit source]
Spina bifida (or myelodysplasia) is a congenital condition where there is failure of the neural tubes to close during foetal development.[1] The neural tube is the embryonic structure that develops into the spinal cord and brain. Spina bifida ranges in terms of severity.[2][3] Lesions most commonly occur in the lumbar regions but they can occur in all parts of the spine.[2]
Types of Spina Bifida[edit | edit source]
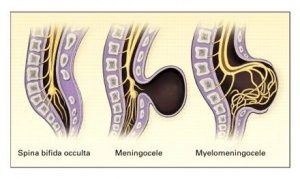
Spina bifida is divided into spina bifida occulta and spina bifida aperta (see image below).[1]
Spina bifida occulta[1]
- closed spinal dysraphism
- most mild form
- there is a hidden vertebral defect
Spina bifida aperta[1]
- open spinal dysraphism
- examples of spina bifida aperta:
- meningocele: the meninges are involved[4]
- meningomyelocele: the lesion involves parts of the spinal cord and the meninges within the sac[4]
- myeloschisis: the neural tissue is exposed to the environment, without a sac or meninges[5]
Embryology[edit | edit source]
Neural tube defects occur early in pregnancy, between day 17 and day 30 gestation. This defect disrupts the overlying tissues, thus preventing the vertebral arch from closing.[6] If the posterior vertebral arch and overlying tissues do not form normally, the spinal cord and meninges may then herniate out through the defect, resulting in meningomyelocele (MMC).
Spina bifida occulta occurs if the vertebral arch fails to grow and fuse normally but the spinal cord and meninges are not disturbed.[3]
Pathophysiology[edit | edit source]
In MMC, the development of the cranial neural tube is affected. This causes various central nervous system changes, including Chiari type II malformation, which is a structural change in the cerebellum. Chiari type II malformation is associated with cerebellar hypoplasia and descent of the lower part of the brainstem into the upper cervical canal. This affects the movement and absorption of cerebrospinal fluid and results in hydrocephalus (please note that hydrochephalus affects >90% of infants with MMC).[3] MMC can also cause spine malformations, hydronephrosis, cardiac defects, and gastrointestinal anomalies.[3]
Epidemiology[edit | edit source]
The incidence of spina bifida worldwide 18.6 per 10,000.[7] The most common open neural tube defect is myelomeningocele.[8]
- There are within country differences between racial and ethnic groups[9]
- there are higher rates of spina bifida in Hispanic groups in the United States
- there is a lower prevalence in African-Americans
- The risk of myelomeningocele in females is 3-7 times higher than males
- There are higher rates of myelomeningocele in China, some parts of Africa, the Middle East, Thailand, India
- it has been noted that different guidelines on folate supplementation may have an impact on these variations in rates[8]
Aetiology[edit | edit source]
Several risk factors have been linked to neural tube defect, including:[9]
- Chromosomal and genetic conditions:
- parents or siblings with neural tube defect increases the risk
- trisomies 13 and 18
- HARD syndrome (Hydrocephalus, agyria and retinal dysplasia)
- Maternal environmental factors and exposure:
- alcohol use
- caffeine intake
- smoking, air pollution
- disinfectant by-products found in drinking water
- exposure to organic solvents, pesticides, nitrate-related compounds, polycyclic aromatic hydrocarbons, fumonisins
- maternal fever or hyperthermia (especially in the first trimester) from febrile illness or external sources like a sauna, or hot tub[3]
- Maternal medical conditions:
- women with low red blood cell folate levels during early pregnancy have up to a 6 times greater risk of having a child with a neural tube defect
- elevated glycemic index, and gestational diabetes mellitus
- infections
- obesity
- stress
- maternal diarrhoea[10]
- Maternal nutritional deficiencies:
- folate
- methionine
- zinc
- vitamin C
- vitamin B12
- choline
- Maternal medications:
- intrauterine exposure to antiepileptic drugs, particularly valproate and carbamazepine
- drugs used to induce ovulation
- various folic acid antagonists
Diagnostic Procedures[edit | edit source]
Diagnosis of MMC in a newborn is usually obvious because there is a visible bulge in the back, which consists of the protruding membrane-covered sac containing meninges, cerebrospinal fluid and nerve tissue. The clinical features of MMC depend on the:
- level of involvement
- presence of hydrocephalus
- associated brain abnormalities
Testing options in the foetus include:[3][11]
- maternal serum α-fetoprotein (MSAFP) screening test
- abnormally elevated levels can suggest (but not confirm) the baby has spina bifida / a neural tube defect
- detailed ultrasonography (diagnosis is usually more accurate in the second trimester[11])
- ultrasound scans will diagnose 92% of neural tube defects
- mothers with elevated MSAFP levels and a normal-appearing ultrasound scan may be evaluated by amniocentesis for the presence of elevated acetylcholinesterase levels in the amniotic fluid[3]
Management / Interventions[edit | edit source]
Generally, infants with MMC have surgery to close the spinal cord defect soon after birth.[3][11] Additional surgeries may be required to manage other issues in the feet, hips, or spine. Individuals with hydrocephalus will also require a shunt to be placed (and subsequent surgeries for the shunt). Surgery may also be required to help prevent issues such as weakness and bladder / bowel dysfunction. Catheterisation may be necessary to help preserve bladder function.[3][11]
Management of Myelomeningocele Study (MOMS):[12] The MOMS trial was a multicentre clinical trial sponsored by the National Institutes of Health in the United States. It began in 2002 and was designed to determine the best treatment for MMC - it compared foetal surgery and surgical repair after birth. It was found that prenatal surgery:[13][12]
- reduces midbrain herniation
- decreases the need to shunt cerebrospinal fluid from the brain
- had a positive impact on mental development / motor function
- improved the likelihood that a child would be able to walk unaided
The MOMS trial, therefore, supports the use of foetal surgery as an effective option for MMC.[12] It found that some of the factors associated with Chiari type II malformation and hydrocephalus develop during the second half of pregnancy. Therefore, early surgery may enable some restoration of nerve function in pregnancy and potentially reverse the development of MMC.[13]
Rehabilitation[edit | edit source]
A multidisciplinary approach is essential for successful outcomes in individuals with MMC. An individual should be assessed as soon after birth as possible. The focus of rehabilitation will change with the changing needs of the patient. Regular review is essential ensure rehabilitation continues to meet the patient's needs. Parents / caregivers should be involved in patient care.
Clinical Presentation[edit | edit source]
Possible signs include:[3]
- flaccid or spastic paralysis of the lower limbs
- urinary and / or faecal incontinence
- hydrocephalus
- poor trunk control
- musculoskeletal complications
- scoliosis
- hip dysplasia
- hip dislocation
- hip / knee contracture
- clubfoot
- muscle atrophy
Physical Assessment[edit | edit source]
During the physical assessment, assess for:
- open wound
- deformities
- skin abnormalities
- sensation
- changes in muscle tone
- changes in muscle strength
- changes in range of motion
- contractures
- dislocation
- developmental milestones
Plan of care[edit | edit source]
The plan of care will include:
- preventing / correcting deformity
- maintaining / improving physiological properties of joints and muscles
- monitoring normal motor development
- educating parent(s), caregivers
- encouraing and maximising independent mobility
- encouraging participation in regular physical activity
Means of Treatment[edit | edit source]
Treatments might include:
- serial casting
- passive mobilisation, graded exercises and stretches
- tactile stimulation
- balance and trunk control exercises
- positioning
- orthotics and assistive devices
- parent / caregivers education: parents or caregivers should be educated about the child’s condition, progress, and prognosis and be involved in treatment planning and home programmes[14]
General Complications[edit | edit source]
Complications of MMC include the following:[3]
- reproductive organs impairment
- only 5.0% to 7.5% of the MMC population have a normal urologic function
- neurogenic bowel: approximately 10% of children with MMC have bowel continence
- musculoskeletal complications
- psychosocial issues: vulnerable child syndrome
- pressure sores
- learning disabilities
Neurosurgical Complications[edit | edit source]
There are specific neurosurgical complications that should be considered:
- wound infection rates range from 7% to 12%[3]
- hydrocephalus
- visual impairment
- ventriculitis
- shunt failure
- Chiari compression
- 5-32% of infants with MMC present with signs of Chiari compression
- it is the most common cause of death in patients with MMC
- Chiari compression can occur at any time, but if it occurs in the first year of life it is associated with a mortality rate of up to 50%[15]
- chronic headaches (the most frequently reported symptom)[15]
- obesity
- common in children with MMC
- the higher the lesion, the higher the percentage of body fat[3]
- in children with L1–L3 lesions, increasing obesity is associated with decreased ability to ambulate[3]
- children with MMC tend to reach peak ambulation at around 10 years
- this is followed by a gradual decrease in function over the following 10 years
- children who ambulate more have been found to have a lower percentage of body fat[3]
References[edit | edit source]
- ↑ 1.0 1.1 1.2 1.3 Brea CM, Munakomi S. Spina Bifida. [Updated 2023 Feb 12]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK559265/?report=classic
- ↑ 2.0 2.1 Lundy-Ekman L (2007). Neuroscience: Fundamentals for Rehabilitation. 3rd edition. St. Louis: Saunders, 2007
- ↑ 3.00 3.01 3.02 3.03 3.04 3.05 3.06 3.07 3.08 3.09 3.10 3.11 3.12 3.13 3.14 Spina Bifida: Background, Pathophysiology, Etiology [Internet]. Emedicine.medscape.com. 2019 [cited 2 March 2019]. Available from: http://emedicine.medscape.com/article/311113-overview
- ↑ 4.0 4.1 Burke R, Liptak G. Providing a Primary Care Medical Home for Children and Youth With Spina Bifida. PEDIATRICS. 2011;128(6):e1645-e1657.
- ↑ Chatterjee S, Dasgupta A. Myeloschisis . In: Alexiou, G, Prodromou N. (editors). Pediatric Neurosurgery for Clinicians. Springer: Cham, 2022.
- ↑ Fletcher JM, Copeland K, Frederick JA (2005). Spinal lesion level in spina bifida: a source of neural and cognitive heterogeneity. Journal of Neurosurgery. 102(3 Suppl):268-79
- ↑ Hassan AE, Du YL, Lee SY, Wang A, Farmer DL. Spina Bifida: A Review of the Genetics, Pathophysiology and Emerging Cellular Therapies. Journal of Developmental Biology. 2022 Jun 6;10(2):22.
- ↑ 8.0 8.1 Sahni M, Alsaleem M, Ohri A. Meningomyelocele. [Updated 2023 Feb 5]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK536959/
- ↑ 9.0 9.1 Copp AJ, Adzick NS, Chitty LS, Fletcher JM, Holmbeck GN, Shaw GM. Spina bifida. Nat Rev Dis Primers. 2015 Apr 30;1:15007.
- ↑ Canfield M, Ramadhani T, Shaw G, Carmichael S, Waller D, Mosley B et al. Anencephaly and spina bifida among Hispanics: maternal, sociodemographic, and acculturation factors in the National Birth Defects Prevention Study. Birth Defects Research Part A: Clinical and Molecular Teratology. 2009;85(7):637-646.
- ↑ 11.0 11.1 11.2 11.3 National Institute of Neurological Disorders and Stroke. Spina Bifida. Available from: https://www.ninds.nih.gov/health-information/disorders/spina-bifida (last accessed 15 May 2023).
- ↑ 12.0 12.1 12.2 Farmer DL, Thom EA, Brock JW 3rd, Burrows PK, Johnson MP, Howell LJ, et al. The Management of Myelomeningocele Study: full cohort 30-month pediatric outcomes. Am J Obstet Gynecol. 2018 Feb;218(2):256.e1-256.e13.
- ↑ 13.0 13.1 University of California San Francisco. Spina Bifida MOMS Trial. Available from: https://fetus.ucsf.edu/spina-bifida-moms-trial/ (last accessed 13 May 2023).
- ↑ McDonnell G, McCann J. Issues of medical management in adults with spina bifida. Child's Nervous System. 2000;16(4):222-227.
- ↑ 15.0 15.1 Campbell, SK, Linden, DW, Palisano RJ (2000). Physical Therapy for Children (2nd Edition). Philadelphia, PA: W.B. Saunders.