Sturge-Weber Syndrome
Original Editors - Marti Bradbury & Kayla Stull from Bellarmine University's Pathophysiology of Complex Patient Problems project.
Top Contributors - Kayla Stull, Marti Bradbury, Elaine Lonnemann, Admin, Kim Jackson and Naomi O'Reilly
Definition/Description[edit | edit source]
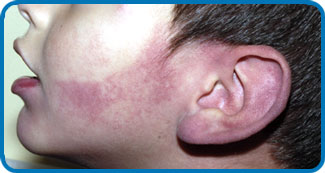
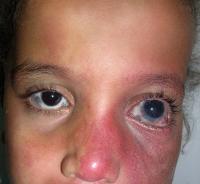
Sturge-Weber Syndrome (SWS), also known as encephalotrigeminal angiomatosis, is a sporadic neurocutaneous disorder that affects the meninges (most often the pia mater and acrachnoid mater) of the brain and the skin of the face. Involvement is normally unilateral, but may be bilateral. The disease is caused by embryonic blood vessels that fail to regress at the appropriate time of development. This leaves residual blood vessels that result in the formation of angiomas on the face, in the meninges, and in the ipsilateral eye. The angiomas of the face are referred to as port wine stains and are most often present in the opthalmic and maxillary divisions of the trigeminal nerve.[1]
Those affected by the disorder may have involvement of both the CNS and the skin of the face or they present with only one area of involvment. The Roach Scale is used to classify the disorder into three types, based on the areas that are affected by the residual blood vessels.
- Type I: Angiomas are present in both the skin of the face and meninges, ipsilateral eye is also typically affected
- Type II: Angioma only present on the face, ipsilateral eye may be affected
- Type III: Angioma only present in the meninges, ipsilateral eye is usally not affected[2]
Hypertrophy of the tissue directly beneath the angioma is typically present. This may lead to assymetries in facial features in addition to the port wine stain. Hypertrophy may also also lead to calcification within CNS resulting in neurological symptoms that include seizures, focal deficits, headaches, and developmental delays.[1]
The neurological symptoms of SWS are progessive in nature.[3] This is most commonly attributed to the "vascular steal phenomenon" in which the residual blood vessels steal blood from the rest of the cortex resulting in hypoxic injury to CNS tissue and increased calcification around the angioma. This leads to increased risk for seizures and neurological deficits.[1]
Prevalence[edit | edit source]
The incidence of SWS is estimated to be 1 per 50,000. Race has not been reported to have an influence on the prevalence of the disorder.[4] Both sexes have been documented to be affected equally.[1]
Characteristics/Clinical Presentation[edit | edit source]
As it was stated before, SWS primarly affects three areas - the skin of the face, the meninges of the brain, and the eye. Each of these areas can be affected in a number of different ways. The possible manifestions of the disease are described below.
Manifestions of the Face:
- Port Wine Stains most often present in the distribution of the trigeminal nerve. Typically the opthalmic and maxillary divisions are most affected, while the mandibular portion is affected less often. Port wine stains associated with SWS are often progressive. They begin as a light pink color at birth and then become a dark red or purple color as the disease progresses. Port wine stains are not always isolated to the face and may be present on other areas of the body. The presence of a port wine stain alone does not indicate SWS. It is necessary to differeniate between SWS and other cutaneous disorders that may present similar skin pigmentation abnormalities.[5]
Manifestations of the CNS:
- Seziures can be attributed to ischemia of the cortex and irritation of the brain secondary to calfication. Most seizures begin within the first 24 months of life and indicate a higher risk for developmental delay than those who do not have seizures. Earlier onset may be seen in those with bilateral involvement. Seizures are normally partial and focal due to the focal nature of the angioma associated with SWS. Seizures can often be at least partially controlled with medication. [5]
- Hemiparesis may occur if CNS damage is present in an area of the brain responsible for motor control. The specific location of the damage will determine that areas of the body that are affected and to what extent. Hemiparesis is often accompanied by a migraine headache indicating a vascular problem.[7]
- Developmental delay may occur as result of decreased blood supply to the cortex. The severity of developmental delay is based upon the amount of neurological damage. Earlier onset of seizures generally indicates a greater risk for developmental delay. Learning disorders and attention deficit hyperactivity disorder may also accompany developmental delays.[4]
- Headaches tend to develop because of vascular disease. Headaches are typically described migraine-like and can be debilitating. Onset of headaches varies based on the progression of the disease. Onset at <10 years of age is a common finding in children with SWS.[5]
Manifestations of the Eye:
- Glaucoma normally develops in the ipsilateral eye only when the eyelid is affected by the port wine stain (opthalmic portion of the trigeminal nerve). Occasionally, bilateral glaucoma my occur in cases of bilateral vascular abnormalities. Glaucoma tends to development either in the first year of life or between the ages of 5-9.[5]
- Blindness may result if glaucoma is left untreated. Elevated intra-ocular pressure levels may lead to damage to the optic nerve. Visual deficits may present as feild cuts or total blindness.[5]
- Buphthalmos or enlargement of the eye may occur secondary to the hypertrohy is often present beneath areas of port wine stain.[5]
Associated Co-morbidities[edit | edit source]
There are no significant co-morbidities associated with Sturge-Weber syndrome outside of the primary manifestations of the disorder.
Medications[edit | edit source]
Medications used to treat SWS will vary greatly depending the presentation of symptoms in those with the disorder. Medical Intervention can include any of the following:
- Anticonvulsants work to discontinue electrical siezure activity quickly and reduce the likelihood of siezure reoccurance. Examples of these medications include Carbamazepine (Tegretol), Phenytoin (Dilantin), Valproic acid (Depakote, Depakene, Depacon), Gabapentin (Neurontin), Lamotrigine (Lamictal), as well as many others.[8]
- Beta Blockers are used to help decrease intraoccular pressure by reducing the amount of aqueous humor produced in the eye. Aqueous humor is a plasma-like substance largely composed of protiens which help support and nourish occular tissue. Aqueous humor also assists in maintaining appropriate intraoccular pressure but in those with SWS this substance is overproduced. Levobunolol 0.25% or 0.5% (Betagan) is a beta blocker commonly used in SWS. [8]
- Carbonic Anhydrase Inhibitors lower intraoccular pressure in much the same way that beta blockers do, by reducing production of aqueous humor. These drugs include Dorzolamide 2% (Trusopt) and Brinzolamide 1% (Azopt).[8]
- Prostaglandin Analogues also work to lower intraoccular pressure, though instead of decreasing aqueous production directly they, instead, increase the outflow of the fluid away from the eye through the proper pathway. This pathway is known as the uveoscleral pathway and is located inferior to the eye. Latanoprost 0.005% (Xalatan) is a prostaglandin analogue used for this purpose.[8]
- Topical Corticosteroids are used to treat occular symptoms. Prednisolone acetate 1% inhibits the edema, fibrin deposition,capillary dilation and phagocytic migration during the acute inflammatory response. Dexamethasone ophthalmic (Maxidex, Ozurdex) and Triamcinolone (Triesence) work by suppressing the migration of polymorphonuclear leukocytes and reversing capillary permeability. [8]
- Antineoplastic Agents work by inhibiting DNA synthesis in order to decrease or stop cell growth and proliferation. Two examples of antineoplastic agents are Fluorouracil (Efudex) and Mitomycin. [8]
Diagnostic Tests/Lab Tests/Lab Values/Imaging[edit | edit source]
The diagnosis of Sturge-Weber Syndrome is most commonly made by the observation of a facial port wine stain in combination with abnormal blood vessels on the suface of the brain and/or glaucoma. Diagnosis is typically made at birth. The following lab tests and imaging techniques can be used to diagnose SWS.
Lab Tests:
- Cerebrospinal Fluid Analysis may show elevated protein levels. It is thought that protein may be elevated because of microhemorrhage that may occur within the brain.[9]
Imaging:
- Radiographs of the skull may show "tram-track" calcification. These calcifications are located between the arachnoid and the pia mater. The follow the pattern of the gyri in a curvilinear and parallel pattern. The calcifications are predominately seen in the parietal and occipital lobes. In more severe cases, the frontal lobe may also be involved and the calcifications may be seen bilaterally.[10] Califications are not present initially and will not be seen on early radiographs. For this reason, plain radiographs are no longer used to diagnose the disease but rather to determine the severity and progression of the disease.[9]
- Angiography will illustrate the abnormal vasculature associated with SWS. Most abnormalities are seen within the venous system.[9]
- CT Scan will show early calcification in infants. Other abnormal findings on CT scans may also include brain atrophy secondary to a lack of normal blood flow, choroid plexus enlargement due to the inability to aquately transport CSF, and a breakdown of the blood-brain barrier during seizures.[9]
- MRI will allow for early diagnosis of SWS because the images will show the formation of the angioma and early venous abnormalities. MRI may also show increased myelination in the area of the angioma, an enlarged choroid plexus, atrophy of cortical tissue (predominately white matter), and abormalties in the corticospinal tracts when hemiparesis is present.[9]
- Single-photon Emission Commuted Tomography (SPECT) measures cerebral blood flow and will demonstrate a lack of adequate blood flow in the area of the angioma. Underperfusion of the cortex is one of the earliest signs of SWS. It is typically present before calcification develops or the onset of seizures. SPECT will also illustrate areas of ischemia within the cortex during seizures.[9]
- Positron Emission Tomography (PET) will identify metabolic abnormalities within the affected hemisphere.[9]
Other Tests:
- Electroencephalogram (EEG) is used to measure electrical activity of the brain through the use of surface electrodes placed on the scalp.[11] In the presence of SWS, EEG findings typically include less electrical current on the affected side. The difference in electrical activity between the affected and unaffected side can be useful in determining the severity of disease. When multiple tests are performed over time, progression of the disease can also be evaluated.[12]
- Transcranial Doppler Ultrasonagraphy is used to evaluate cortical blood flow. Decreased blood flow velocity is often found in the middle and posterior cerebral arteries in chilldren with SWS. This may explain the hypoperfusion associated with the disorder.[9]
Etiology/Causes[edit | edit source]
Though it is not believed to be hereditary in nature, this is a congenital disease which develops in utero and manifests at birth. Because of the lack of knowledge surrounding its cause, prevention of this syndrome is impossible.[13]
Systemic Involvement[edit | edit source]
Sturge-Weber Syndrome does not typically present with impairments or abnormalities in systems outside of the following five.
Integumentary
- The signiture sign of SWS is a port wine stain that presents unilaterally in the face of the majority of patients. This mark is caused by swollen blood vessels that cause the skin to become pinkish-purple color. In most patients, the mark covers the forehead and eyelid but can sometimes extend into the rest of the face, even crossing midline to become bilateral. It is estimated that 96% of SWS patients have some kind of port wine stain.[14] This is a sign that is immediately noticeable at birth and can darken over time. While it is uncommon, the facial port wine stain can also fade over time to the point where it can no longer be seen. It is important to note that while this is a common sign of SWS, the existance of a port wine stain does not mean an individual will have this syndrome. These markings can exist outside of a SWS diagnosis.
- Swelling of the lips, throat, and gums can become a problem due to the port wine stain. This swelling can cause the patient to experience issues with tooth decay and bleeding of the gums.[14][15]
Neurological
- Venous Angiomatosis is almost always present meaning these patients develop angiomas within the venous capillaries of the meninges. The occipital and parietal lobes of the hemisphere ipsilateral to the facial port-wine stain are most commonly involved although it is not impossible for angiomatosis to also occur bilaterally as well as advance to the frontal and temporal lobes as the patient ages and the disease progresses[14][16]
- Damage to the cerebral cortex due to the angiomas and associated tissue calcification of brain is believed to be linked with seizure activity which is one of the most common neurological features of SWS patients. These seizures can be focal or generalized affecting approximately 85% of people living with this diagnosis. Convulsions generally present on the side of the body opposite the facial port wine stain. It is critical that individuals be diagnosed as early as possible in order to treat these seizures with the appropriate medical intervention; if left undiagnosed and untreated these seizures can cause permanent damage to the soft tissues of the brain resulting in further deficits.
- Mental Retardation is common in individuals with SWS. Mental delay becomes more significant when seizure activity is noted within the first year of life and is likely to continue to advance in severity if seizure treatment is not sought or is resisted.[14][15]
Neuromuscular
- Due to atrophy of the cortical areas in the hemisphere of the brain ipsilateral to the facial port wine stain, these individuals can experience muscle weakness and even hemiparesis contralateral to the facial mark. Hemiparesis most commonly occurs when an individual has a series of seizures or seizures of increased intensity. Muscular weakness can be intermittant but is often progressive with the progression depending on the amount of cortical atrophy present in the brain.[17]
Occular
- Glaucoma is a problem that can effect these individuals at any point in their lives. It can be present at birth or later in life and is likely to lead to vision changes due to damage of the optic nerve. If severe enough blindness can occur. [15]
Endocrine
- Involvement of brain structures controlling hormone regulation is possible in patients with SWS, however problems associated with these structures are much more rare than involvement of more superficial cortical structures. If a patient does present with deficits in his or her endocrine system, he or she can have difficulty with regulation of growth hormone, thyroid hormone, cortisol, estrogen, testosterone, or anti-diuretic hormone. It is possible that these regulatory and/or production impairments can be resolved or improved through hormone-replacement therapies.[18]
Medical Management (current best evidence)[edit | edit source]
Because the presentation of SWS can vary greatly between children, medical treatment is based upon the symptoms of each child. The medical treatments available for the various aspects of SWS are addressed below.
Treatment of Port Wine Stain
Laser treatment is used to reduce port wine stains in children with SWS. Multiple treatments given over a period of several months is effective in decreasing the prominence of port wine stains. Most often a Flashlamp-Pulsed Tunable Dye Laser is used to target the abnormal vascular structure beneath the skin.[18] This type of laser has been effective reducing port wine stains in children of all ages and over all areas of the face. Children under the age of 7 and port wine stains over bony prominences typically require fewer treatments than those in older children or over the fleshy part of the cheek. In most cases, affected skin is identical in color and texture to adjacent skin after treatment. A small percentage of individuals may have resulting hyperpigmentation or small depressed scars over the area of the port wine stain post-treatment.[19]
Treatment of Headaches
Headaches are typically treated with medication if they interfere with daily activities of the child. Headaches may also occur in accordance with seizures. In this case, it is important to treat the seizure the relieve the headache pain.[18]
Treatment of Epilepsy
In most cases, antiepileptic drugs are effective in treating seizures. In more severe cases of SWS, children may experience uncontrolled and life threatening seizures despite medication. These children may require surgery to remove the areas of brain tissue responsible for the epilepsy.[18] Surgical options include a complete hemispherectomy or a focal resection - determination of most appropriate surgical option is based upon the amount and location of epileptic tissue. The age of the child at time of surgery does not effect the reduction in seizure activity, however, surgery at an early age may porduce more developmental gains than surgery at a later age.[20]
Treatment of Glaucoma
Medications and/or eye drops are prescribed to promote normal intra-ocular pressure in the affected eye(s) to prevent optic nerve damage. There are several types of medications that may be prescribed to either decrease the amount of aqueous fluid that is produced or promote drainage of the fluid out of the eye. The specific drugs are addressed in the section labeled "Medications." If use of the medications fails to result in maintainence of normal intra-ocular pressure, surgery may be required.[18][21] Much like pharmacological interventions, the goal of surgery is to either decrease the production of fluid in the eye or increase the outflow of fluid from the eye. Several different surgical procedures may be used to correct glaucoma in children with Sturge Weber Syndrome. These include goniotomy, trabeculotomy, trabeculectomy, tube-shunt, and cyclodestructive procedures. Both the goniotomy and trabeculotomy are used to correct the anatomical abnormalties that may be associated with glaucoma. In both procedures, an opening is created in the trabecular meshwork of the eye so that fluid may drain normally. Goniotomy is performed from the interior apect of the eye and requires the presence of a clear cornea, while trabeculotomy is performed from the exterior surface and may be completed even if the individual presents with an abnormal cornea. Both procedures have demonstrated equal effectiveness in the treatment of glaucoma associated with SWS. The goal of a trabeculectomy is to create an alternative pathway for fluid to leave the eye. An opening is created under the conjunctiva that allows fulid to move away from the eye. This surgery has a high failure rate becuase the healing tendencies of children tend to result in closure of the new opening. In the case of failure, a tube-shunt may be placed in the eye that leads to reservoir under the conjunctiva where fluid can be dispersed. If the aforementioned procedures are unsuccessful, surgery may be performed to decrease the production of fluid within the eye. These are know as cyclodestructive porcedures in which the ciliary body is either frozen or rendered ineffective with laser treatment and a decrease in the production of aqueous fluid results.[22]
Physical Therapy Management (Current Best Evidence)[edit | edit source]
The majority of physical therapy intervention for SWS is focused on impairments associated with hemiparesis and muscle weakness. Physical therapy consultations are most often sought during childhood before the patient has full speech and ambulation ability.[23] Treatment of various impairments are most commonly targeted utilizing the following techniques.
Muscle Strengthening/Resistive exercise is utilized in the treatment of SWS to reduce the weakness caused by atrophy in the cortical areas of the brain.
Orthosis education/spasticity management can sometimes be required for those who have spasticity associated with their diagnosis. Due to the fact that most neurological involvement associated with SWS is localized primarily to the cortical regions of the brain, spasticity is not commonly seen is these patients.[23]
CIMT (constraint-induced movement therapy) is a treatment option which involves restricting the motion of one extremity in order to promote the use of the contralateral extremity. This can be a useful technique for more severe cases of SWS that involve hemiparesis. It is rare that CIMT is used in the adult population living with SWS; CIMT is, instead, used during childhood to promote use of the involved extremities and prevent neglect. Parameters for the use of CIMT intervention vary with some patients responding well to one round of treatment and others requiring multiple rounds of CIMT treatment to produce results.[23] CIMT treatment is not used in all SWS patients with hemiparesis, particularly if other treatment methods have proven effective in promoting acheivement of the same goals targeted by CIMT.
Alternative/Holistic Management (current best evidence)[edit | edit source]
Medicinal Marijuana (cannabis oil) is often prescribed to patients who suffer from neurological issues and to those who battle glaucoma on a regular basis. Marijuana has the potential to present less adverse affects when compared to more mainstream medications, but must be administered judiciously as they can effect heart rate and blood pressure almost immediately upon use.[24]
Marijuana has been found to have a positive affect on reducing intraoccular pressure within the eye, which is generally increased in patients with glaucoma. As for the treatment of seizures, it is inconclusive if marijuana can be an effective alternative to more traditional anti-seizure medications. Many patients have found relief through the use of marijuana but it remains unclear if this alternative medication has widespread seizure use or can only target specific seizure types such as partial or tonic-clonic.[24]
The use of medicinal marijuana remains a controversial issue and is often only prescribed as a last resort. The exact scientific reason for its effects are not currently known which adds to its controversy.
Differential Diagnosis[edit | edit source]
1. Klippel-Trenaunay-Weber syndrome consists of port wine stains in both the extremities and the face, as well as, hypertrophy of bone and soft tissue along with the clinical manifestations of Sturge-Weber syndrome. The formation of solid visceral tumors primarily the kidney, adrenal gland, or liver helps to differentiate Klippel-Trenaunay-Weber syndrome from Sturge-Weber syndrome.[25]
2. Beckwith-Wiedemann syndrome consists of facial port wine stain and a number of other visceral symptoms which is useful in distinguishing this disorder from Sturge-Weber syndrome.[25]
3. Dyke-Davidoff-Masson syndrome exists when significant atrophy is occurs in one hemispere during infancy. It may be difficult to differentiate between this disorder and Sturge-Weber syndrome in the absence of a clearly defined port wine stain.[25]
4. Siderosis also results in atrophy of one cerebral hemisphere that is similar to that seen in Sturge-Weber syndrome. Imaging studies (MRI with contrast) that outline cerebral vasculature will be necessary to differentiate between the disorders. MRI will show normal vascular structures with siderosis while abnormal results with be seen with SWS.[25]
Case Reports/ Case Studies[edit | edit source]
Sturge-Weber syndrome: a case report[26]
Sturge-Weber syndrome: A case report[27]
http://www.jpad.org.pk/april%20-%20june%20%202006/12sturge-weber%20syndrome.pdf
Resources
[edit | edit source]
The Sturge-Weber Foundation [28]
Sturge-Weber Syndrome Community [29]
References[edit | edit source]
- ↑ 1.0 1.1 1.2 1.3 Medscape Reference: Drugs, Diseases, and Procedures. Pediatric Sturge-Weber Syndrome: Overview. http://emedicine.medscape.com/article/1177523-overview (accessed 12 March 2012).
- ↑ Gill NC, Bhaskar N. Sturge-Weber Syndrome: A case report. Contemp Clin Dent; 1(3):183-185.
- ↑ Aylett SE, Neville BG, Cross JH. Sturge-Weber syndrome: cerebral haemodynamics during seizure activity. Dev Med Child Neurol 1999;41(7):480-5.
- ↑ 4.0 4.1 Thomas-Sohl KA, Vaslow DF, Maria BL. Sturge-Weber Syndrom: A review. Ped Neurology 2004;30(5):303-310.
- ↑ 5.0 5.1 5.2 5.3 5.4 5.5 Medscape Reference: Drugs, Diseases, and Procedures. Pediatric Sturge-Weber Syndrome: Clinical Presentation. http://emedicine.medscape.com/article/1177523-clinical (accessed 12 March 2012).
- ↑ Miami Children's Hospital. Vascular Birthmarks: PWS (Port Wine Stain). http://www.mch.com/page/EN/2702/Vascular-Birthmarks/PWS-(Port-wine-stain).aspx (accessed 17 April 2012).
- ↑ Jung A, Raman A, Rowland Hill C. Acute hemiparesis in Sturge-Weber syndrome. Pract Neurol 2009;9(3):169-71.
- ↑ 8.0 8.1 8.2 8.3 8.4 8.5 Medscape Reference, Sturge-Weber Syndrome. Medications. http://emedicine.medscape.com/article/1219317-medication#1 (accessed 1 Mar 2012).
- ↑ 9.0 9.1 9.2 9.3 9.4 9.5 9.6 9.7 Medscape Reference: Drugs, Diseases, and Procedures. Pediatric Sturge-Weber Syndrome: Work-up. http://emedicine.medscape.com/article/1177523-workup (accessed 12 March 2012).
- ↑ Akpinar, E. The Tram-Track Sign: Cortical Calcification. Radiology 2004;231:515-516.
- ↑ Medline Plus: Trusted health information for you. EEG. http://www.nlm.nih.gov/medlineplus/ency/article/003931.htm (accessed 9 March 2012).
- ↑ Hatfield LA, Crone NE, Kossoff EH, Ewen JB, Pyzik PL, Lin DD, et al. Quantitative EEG Assymetry Correlates with Clinical Severity in Unilateral STurge-Weber Syndrome. Epilepsia 2007;48(1):191-195.
- ↑ BBC Health: Physical Health. Sturge Weber Syndrome. http://www.bbc.co.uk/health/physical_health/conditions/sturgeweber2.shtml#causes_and_risk_factors_ (accessed 15 March 2012).
- ↑ 14.0 14.1 14.2 14.3 14.4 Medscape Reference, Sturge-Weber Syndrome. Overview. http://emedicine.medscape.com/article/1219317-medication#1 (accessed 1 Mar 2012).
- ↑ 15.0 15.1 15.2 Sturge-Weber Syndrome: Facts and Information. Disabled world towards tomorrow. http://www.disabled-world.com/health/neurology/sturge-weber-syndrome.php. (accessed 28 Mar 2012).
- ↑ Miyama S, Goto M. Leptomeningeal Angiomatosis with infantile spasms. Ped Neuro 2004; 31(5): 353-6. http://www.sciencedirect.com/science/article/pii/S0887899404002887 (accessed 29 Mar 2012).
- ↑ Medlink neurology: clinical summary. Sturge-Weber syndrome.http://www.medlink.com/medlinkcontent.asp (accessed 29 Mar 2012).
- ↑ 18.0 18.1 18.2 18.3 18.4 Hunter Nelson Sturge-Weber Center at Kennedy Krieger Institute. Sturge-Weber Syndrome Center: Treatment of SWS. http://sturgeweber.kennedykrieger.org/treatment.jsp (accessed 2 April 2012).
- ↑ Tian Tan O, Sherwood K, Gilchrest BA. Treatment of children with port wine stains using the Flashlamp-Pulsed Tunable Dye laser. N Engl J Med 1989;320:416-421.
- ↑ Bourgeois M, Crimmins DW, De Oliveira RS, Arzimanoglou A, Garnett M, Roujeau T,Di Rocco F, Sainte-Rose C. Surgical treatment of epilepsy in Sturge-Weber Syndrome in children. J Neurosurg Ped 2001;106(1):20-28.
- ↑ Medscape Reference: Drugs, Diseases, and Procedures. Pediatric Sturge-Weber Syndrome: Treatment and Management. http://emedicine.medscape.com/article/1177523-treatment#a1128(accessed 2 April 2012).
- ↑ Glaucoma Research Foundation. Surgical Treatments for Pediatric Glaucoma. http://www.glaucoma.org/treatment/surgical-treatments-for-pediatric-glaucoma.php (accessed 3 April 2012).
- ↑ 23.0 23.1 23.2 Suskauer S, Travato M, Zabel T, Comi A. Physiatric findings in individuals with sturge-weber syndrome. Am J Phys Med Rehabil. 2010; 89(4): 323-30. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3189450/ (accessed 1 April 2012).
- ↑ 24.0 24.1 Gordon E, Devinsky O. Alcohol and marijuana: effects on epilepsy and use by patients with epilepsy. Epilepsia 2001; 42(10):126-72. http://onlinelibrary.wiley.com/doi/10.1046/j.1528-1157.2001.19301.x/full (accessed 1 April 2012).
- ↑ 25.0 25.1 25.2 25.3 Medscape Reference: Drugs, Diseases, and Procedures. Sturge-Weber Syndrome: Differential Diagnoses. http://emedicine.medscape.com/article/1219317-differential (accessed 3 April 2012).
- ↑ Conciecao J, dos Santos L, Sa Bahia T, Silva V, Ranos M, Israel M. Sturge-Weber syndrome: a case report. RSBO 2011 8(4): 469-72. Full version: http://vdisk.univille.edu.br/community/depto_odontologia/get/ODONTOLOGIA/RSBO/RSBO_v8_n4_outubro-dezembrobro2011/v8n4a16.pdf (accessed 25 Mar 2012).
- ↑ Khan H, Afzal M, Anjum F, Javed T. Sturge-Weber syndrome. A case report. J Pakistan Ass Derm 2006; 16:120-22. Full version: http://www.jpad.org.pk/april%20-%20june%20%202006/12sturge-weber%20syndrome.pdf (accessed 25 Mar 2012).
- ↑ The Sturge-Weber Foundation. Welcome. http://www.sturge-weber.org/ (accessed 3 April 2012).
- ↑ Sturge-Weber Syndrome Community. Welcome to the Sturge-Weber Syndrome Community. http://www.swscommunity.org/ (accessed 3 April 2012).