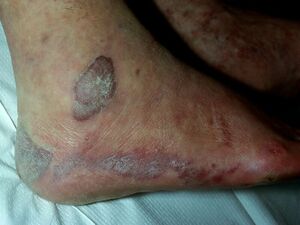
Kaposi Sarcoma
Top Contributors - Carina Therese Magtibay and Ines Musabyemariya
Introduction[edit | edit source]
Kaposi sarcoma (KS) is a soft tissue tumor that affects immunocompromised people including organ transplant recipients and people with acquired immunodeficiency syndrome (AIDs).[1][2]
Moritz Kaposi, an Austro-Hungarian dermatologist, first published "Idiopathisches multiples Pigmentsarkom der Haut" in 1872. He reported several cases of a multifocal pigmented sarcoma of the skin in elderly European men which is now known as Kaposi Sarcoma.[2][3]
Aetiology & Pathological Process[edit | edit source]
KS has a viral aetiology. AIDS epidemic progressed in the 1980s and Human herpesvirus type 8 (HHV-8) in KS lesions was discovered as the causative agent of Kaposi sarcoma in the 1990s[1]. HHV-8 is present in all forms of Kaposi sarcoma and interferes with many normal cell functions and requires cofactors like cytokines or specific proteins to result in the development of Kaposi sarcoma.
After infecting endothelial cells, HHV-8 activates the mTOR pathway, alters the cells to have mesenchymal differentiation, and promotes aberrant angiogenesis. The HHV-8 infected cells can persist and proliferate through immune suppression and inflammation. Expression of latency-associated nuclear antigen (LANA) causes binding of p53 and suppression of apoptosis.[1]
Modes of transmission include:[4]
- Saliva (mainly during childhood, can be transmitted within families) - main mode of transmission.
- Sexual transmission
- Blood transfusion
- Intravenous drug use
Epidemiology & Clinical Presentation[edit | edit source]
In all forms of KS, cutaneous lesions usually present as multiple, pigmented, raised or flat, painless lesions that do not blanch. The earliest cutaneous lesions are often asymptomatic, innocuous-looking, pigmented macules or small papules that vary in colour from pale pink to vivid purple. Larger plaques on the trunk often follow the skin creases as oblong lesions. Occasionally, lesions form exophytic, ulcerated and bleeding nodules that can be associated with painful oedema.[2]
Forms of KS:[1]
- Classic KS
- presents in individuals without HIV infection in older men, typically occurring in elderly men of Mediterranean and Eastern European descent on the lower extremities.
- male: female ratio is 17:1
- occurs primarily in patients over 50 years old of Eastern European and Mediterranean descent.
- at greater risk for secondary malignancies.[5][6]
- Endemic KS
- found in sub-Saharan Africa and has generalized lymph node involvement in children.
- has the predilection for the pediatric population and mirrors HHV-8 seropositivity. The rates of seropositivity in pediatric patients vary extensively throughout Africa, from a low of 2% in Eritrea to almost 100% in the Central African Republic.[7]
- HIV-related KS
- commonly occurring with patients not taking highly active antiretroviral therapy (HAART)
- AIDS-related KS is the second most common tumor in HIV patients with CD4 counts less than 200 cells/mm3 and is an AIDs-defining illness.
- HIV positive male homosexuals have a 5- to 10-fold increased the risk of Kaposi sarcoma.
- Iatrogenic KS
- seen in patients treated with immunosuppressive therapy, especially organ transplant recipients.
- presents with diffuse involvement of the skin and internal organs.
- male: female ratio of 3:1
- Over 5% of transplant patients who develop a de novo malignancy will develop Kaposi sarcoma, a 400- to 500-fold increased risk over the general population.
- Patients with bone marrow or peripheral blood stem cell transplant have much lower risks of developing Kaposi sarcoma compared to solid organ transplant patients.
Diagnostic Procedures[edit | edit source]
Histological Diagnosis[edit | edit source]
Biopsy is required for a definite diagnosis of KS with identical histology across its different epidemiologic forms. The identification and localisation of HHV8 within KS lesional cells using a monoclonal antibody against HHV-8 latent nuclear antigent (LANA) is the most diagnostically helpful immunostaining technique available to differentiate KS from its simulators since it is specific of KS [8]
HHV-8 Diagnostic Tools[edit | edit source]
No other specific HHV-8 tool is routinely used aside from immunohistochemistry for LANA. These diagnostic tools are conducted on an individual basis:
- Serology
- PCR
Differential Diagnosis[edit | edit source]
Histologically, spindle cell vascular lesions in the skin include a differential diagnosis of:[9]
- Interstitial granuloma annulare
- Spindle cell hemangioma
- Acquired tufted angioma
- Kaposiform hemangioendothelioma
- Cutaneous angiosarcoma
- Fibrosarcomatous dermatofibrosarcoma protuberans
- Aneurysmal dermatofibroma
- Acroangiodermatitis
- Spindle cell melanoma
- High-grade sarcomas
The differential diagnosis of Kaposi sarcoma on mucocutaneous surfaces includes:[5]
- Nevi
- Pyogenic granuloma
- Bacillary angiomatosis
- Hemangioma
- Angiosarcoma
- Melanoma
Management / Interventions[edit | edit source]
Local therapies[edit | edit source]
According to European consensus-based interdisciplinary guidelines on the diagnosis and treatment of KS (2019), localized and symptomatic lesions can be treated using local approaches. Currently, no randomized clinical trials are comparing the different local treatment modalities. There are few controlled studies in this area but it is not possible to compare studies, because of the lack of standardized classification systems for disease activity and clinical outcomes.[8]
Local therapies include: [8]
- Radiotherapy - One of the most efficient treatments for all forms of localized KS. Overall response rates range from 47% to 99%
- Surgical excision- Surgical excision is associated with a high recurrence rate so it is indicated only on a few well-defined limited and superficial lesions. Repeated surgical excisions can result to severe functional impairment.
- Cryosurgery and Laser - CO2-Laser and superficial cryotherapy can be temporarily efficient in superficial lesions with 80 to 90% overall response rate. The patient should be informed of the risk of sequelae hypopigmentation.
- Local or intralesional chemical or immune modifying agents
Systemic Treatments[edit | edit source]
The KS type, the extent of the disease, the disease course and symptoms will determine the course of treatment. Systemic treatment is mainly for disease control and symptom relief rather than a cure.[8]
The recommended first-line agents in terms of systemic treatment are:[8]
- Pegylated liposomal doxorubicin
- Paclitaxel
Special Indications per type of KS[edit | edit source]
[edit | edit source]
- Combination antiretroviral therapy (cART) is the first treatment option in HIV-related KS.
- Treatment should be individualized. In most cases, HIV-related KS regresses with cART but systemic chemotherapy is recommended for patients with:
- T1 patients
- rapidly progressive disease
- in the prevention and treatment of immune reconstitution inflammatory syndrome (IRIS)
- Liposomal anthracyclines (fist line) and taxanes (second line) have become the established systemic therapy against KS in combination with cART. It is important to note that KS cannot be eradicated and tumors may recur, leading to patients needing additional therapies.
Post-transplant KS [8][edit | edit source]
- Several studies suggest that in the management of post-transplant KS, tapering down immunosuppressive therapy to the lowest possible level and switching to m-TOR inhibitors such as sirolimus are essential while attempting to keep the allograft functional.
- Although poorly evaluated in post-transplant KS, specific treatments can be useful in patients with extensive or life-threatening lesions in combination with immunosuppressors minimization.
Classic and endemic KS [8][edit | edit source]
- Systemic treatment, although poorly codified, may be required for aggressive forms characterized by:
- lymph node and/or visceral involvement
- severe oedema
- local complications
- rapid extension
- The first options are generally based on the use of liposomal anthracyclines and, less frequently, weekly taxanes.
Physiotherapy Management[edit | edit source]
A holistic approach to treating KS is essential to ensure best patient outcomes. Physiotherapists' roles include improving quality of life and keeping the patient active.[10]
Treatment modalities that can be adopted for the physiotherapeutic management of a patient living with HIV/AIDS: [10]
- Exercise therapy
- Manual therapy
- Compression bandaging and pressure garments
- Gait training
- Chest physiotherapy
- Counseling and health education
References[edit | edit source]
- ↑ 1.0 1.1 1.2 1.3 Bishop BN, Lynch DT. Kaposi sarcoma. InStatPearls [Internet] 2022 Jun 11. StatPearls Publishing.
- ↑ 2.0 2.1 2.2 Cesarman E, Damania B, Krown SE, Martin J, Bower M, Whitby D. Kaposi sarcoma. Nature reviews Disease primers. 2019 Jan 31;5(1):9.
- ↑ Kaposi. Idiopathisches multiples pigmentsarkom der haut. Archiv für Dermatologie und Syphilis. 1872 Jun;4:265-73.
- ↑ Mariggiò G, Koch S, Schulz TF. Kaposi sarcoma herpesvirus pathogenesis. Philosophical Transactions of the Royal Society B: Biological Sciences. 2017 Oct 19;372(1732):20160275.
- ↑ 5.0 5.1 Fatahzadeh M. Kaposi sarcoma: review and medical management update. Oral surgery, oral medicine, oral pathology and oral radiology. 2012 Jan 1;113(1):2-16.
- ↑ Iscovich J, Boffetta P, Franceschi S, Azizi E, Sarid R. Classic Kaposi sarcoma: epidemiology and risk factors. Cancer. 2000 Feb 1;88(3):500-17.
- ↑ Etta EM, Alayande DP, Mavhandu-Ramarumo LG, Gachara G, Bessong PO. HHV-8 seroprevalence and genotype distribution in Africa, 1998–2017: a systematic review. Viruses. 2018 Aug 27;10(9):458.
- ↑ 8.0 8.1 8.2 8.3 8.4 8.5 8.6 8.7 Lebbe C, Garbe C, Stratigos AJ, Harwood C, Peris K, Del Marmol V, Malvehy J, Zalaudek I, Hoeller C, Dummer R, Forsea AM. Diagnosis and treatment of Kaposi's sarcoma: European consensus-based interdisciplinary guideline (EDF/EADO/EORTC). European Journal of Cancer. 2019 Jun 1;114:117-27.
- ↑ Marušić Z, Billings SD. Histopathology of spindle cell vascular tumors. Surgical Pathology Clinics. 2017 Jun 1;10(2):345-66.
- ↑ 10.0 10.1 Adesina MA, Kanmodi KK. Physiotherapeutic management of people living with HIV/AIDS. World News of Natural Sciences. 2018;20.