Opioids: Difference between revisions
Justin Bryan (talk | contribs) No edit summary |
Justin Bryan (talk | contribs) No edit summary |
||
| (14 intermediate revisions by the same user not shown) | |||
| Line 3: | Line 3: | ||
== Introduction == | == Introduction == | ||
[[File:OxyContin branded oxycodone 10mg (OC side).jpg|right|frameless|250x250px]] | [[File:OxyContin branded oxycodone 10mg (OC side).jpg|right|frameless|250x250px]] | ||
An opioid (exogenous opioid) refers to any substance from a group of analgesic agents derived from the | An opioid (exogenous opioid) refers to any substance from a group of analgesic (pain relieving) agents derived from the substance opium. Opioids are a type of depressant, analgesic, drug that slows down the messages being sent through the [[Central Nervous System Pathways|central nervous system]] between the body and the [[Brain Anatomy|brain]]. Although used to treat pain, opioids can entice euphoric feelings and sedative effects which can be addictive. These properties make abuse of this drug group increasingly common. | ||
Opioids can be naturally occurring, synthetic, or semi-synthetic depending how they are derived. Natural and semi-synthetic opioids utilize substances extracted from opium, which is produced from the dried seeds of the opium poppy. Fully synthetic opioids, on the other hand, are derived using laboratory methods to produce the same compounds naturally found in opium through controlled chemical processes. A least 20 different compounds are found in opium; these biologically active substances can either produce analgesic effects directly (i.e. morphine) | Opioids can be naturally occurring, synthetic, or semi-synthetic depending on how they are derived. Natural and semi-synthetic opioids utilize substances extracted from opium, which is produced from the dried seeds of the opium poppy. Fully synthetic opioids, on the other hand, are derived using laboratory methods to produce the same compounds naturally found in opium through controlled chemical processes. A least 20 different compounds are found in opium; these biologically active substances can either produce analgesic effects directly (i.e. morphine) or act as precursors that can be used to produce other analgesic drugs including semi-synthetic opioids.<ref name=":0">Ciccone CD, editors. Pharmacology in Rehabilitation. 5th Ed. Philadelphia: F.A. Davis Company, 2016. p202-218.</ref> | ||
== Clinical Pharmacology == | == Clinical Pharmacology == | ||
Production of opioids begins with four naturally occurring alkaloids which can be isolated from the opium poppy seed (p''apaver somniferum).'' These plant-derived amines, which include morphine, codeine, papaverine and thebaine, can then be used to produce many varieties of semi-synthetic opioids useful in clinical medicine. Common semi-synthetic opioids derived from these four substances include diamorphine, dihydrocodeine, buprenorphine, nalbuphine, naloxone and oxycodone.<ref name=":1">Pathan, H., & Williams, J. (2012). [https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4590096/ Basic opioid pharmacology: an update.] ''British journal of pain'' | Production of opioids begins with four naturally occurring alkaloids which can be isolated from the opium poppy seed (p''apaver somniferum).'' These plant-derived amines, which include morphine, codeine, papaverine, and thebaine, can then be used to produce many varieties of semi-synthetic opioids useful in clinical medicine. Common semi-synthetic opioids derived from these four substances include diamorphine, dihydrocodeine, buprenorphine, nalbuphine, naloxone, and oxycodone.<ref name=":1">Pathan, H., & Williams, J. (2012). [https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4590096/ Basic opioid pharmacology: an update.] ''British journal of pain''; ''6''(1): 11-6.</ref> | ||
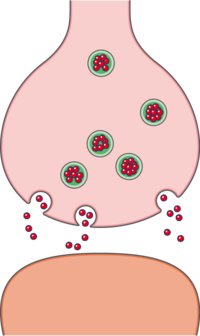
Opioids exert their analgesic properties by binding to specialized "opioid" receptors that already exist on | Opioids exert their analgesic properties by binding to specialized "opioid" receptors that already exist on nerve cells at specific sites in the central and peripheral nervous systems. These receptors exist in concert with endogenous (produced by the body) opioid-like substances, both of which act as part of the body's natural process of controlling pain and [[Inflammation Acute and Chronic|inflammation]]. The three groups of endogenous opioid-like substances include endorphins, enkephalins, and dynorphins. In addition to pain control, endogenous opioid-like substances are believed to also play a role in other processes including response to stress, [[Immune System|immune modulation]], GI function, and eating.<ref name=":0" /> | ||
===== Opioid Receptors ===== | ===== Opioid Receptors ===== | ||
There are three major types of opioid | There are three major types of opioid receptors, mu (𝜇), delta (𝛿), and kappa (𝜅), found throughout the body. Other opioid receptors do exist, i.e. nociceptin/orphanin FQ peptide receptors, however, no known exogenous opioid has been found to specifically act upon them. As such, opioids are commonly classified by their ability to exert an effect by binding to one of the three main receptors (𝜇, 𝛿, and 𝜅). All 3 receptors will produce analgesia when bound by an opioid, but the degree of effect is determined by how strongly a given opioid binds to a given receptor. This degree of "affinity" produces further sub-classifications under each receptor type.<ref name=":0" /><ref name=":1" /> | ||
In addition to producing analgesia, each receptor type also produces a unique and distinct spectrum of additional, and often adverse, effects when bound and activated. This specificity is important when considering patient safety, especially in certain situations, or when pre-existing conditions could be exacerbated by the effects. Common unique effects of each receptor are listed below:<ref name=":0" /> | |||
* Mu (𝜇) - Sedative effects, depression of respiratory function, constipation, reduction in release of neurotransmitters | * Mu (𝜇) - Sedative effects, depression of respiratory function, constipation, reduction in the release of neurotransmitters | ||
* Delta (𝛿) - Sedative effects, constipation, psychoactive effects | * Delta (𝛿) - Sedative effects, constipation, psychoactive effects | ||
* Kappa (𝜅) - Heightened release of hormones, reduction in release of neurotransmitters | * Kappa (𝜅) - Heightened release of hormones, reduction in the release of neurotransmitters | ||
===== Opioid Mechanisms of Action ===== | ===== Opioid Mechanisms of Action ===== | ||
Opioids produce their analgesic effect by acting directly on the synapses of neurons in the brain, spinal cord, and | Opioids produce their analgesic effect by acting directly on the synapses of neurons in the [[Brain Anatomy|brain]], [[Spinal cord anatomy|spinal cord]], and peripheral tissues. Each of these three areas are unique in their characteristics and how opioids specifically exert their effect.<ref name=":0" /> | ||
[[File:Systeme nerveux.png|right|frameless|390x390px|alt=]] | |||
* '''Spinal Cord:''' Opioid action at the level of the spinal cord | * '''Spinal Cord:''' Opioid action at the level of the spinal cord occurs by inhibiting the transmission of pain signals traveling from peripheral nerves to the brain. This occurs when opioids bind to the presynaptic receptors of pain neurons in this area, resulting in a reduction in the release of pain-mediating neurotransmitters including Substance P. | ||
* '''Brain:''' Opioid action in the brain occurs by altering the action of neurons responsible for interpretation and transmission of pain signals. A common belief is that opioids facilitate increased activity of descending pain pathways by blocking the signals of interneurons that would typically inhibit these pathways. When this process occurs it is known as disinhibition. At the same time, increased descending pathway activity also blocks the transmission of pain signals in the spinal cord through the same mechanism as opioids acting on this region. | * '''Brain:''' Opioid action in the brain occurs by altering the action of neurons responsible for the interpretation and transmission of pain signals. A common belief is that opioids facilitate increased activity of descending pain pathways by blocking the signals of interneurons that would typically inhibit these pathways. When this process occurs it is known as disinhibition. At the same time, increased descending pathway activity also blocks the transmission of ascending pain signals in the spinal cord through the same mechanism as opioids acting on this region. | ||
* '''Peripheral Tissue:''' Opioid receptors have been found on peripheral sensory neurons, allowing opioids to act directly in these areas. When the receptors on these peripheral nerves are bound by opioids, the excitability of the neurons | * '''Peripheral Tissue:''' Opioid receptors have been found on peripheral sensory neurons, allowing opioids to act directly in these areas. When the receptors on these peripheral nerves are bound by opioids, the excitability of the neurons is reduced. In this way, analgesia is achieved by preventing pain signals from even being produced. | ||
== Categories of Opioid Drugs == | == Categories of Opioid Drugs == | ||
Opioids are classified based on the characteristics of how they bind to opioid receptors in the body. Specifically, these | Opioids are classified based on the characteristics of how they bind to opioid receptors in the body. Specifically, these classifications are heavily influenced by the strength, or affinity, to which the agent binds. Additionally, the specification of agonist versus antagonist is an important distinction. Agonist agents are defined by their ability to bind and activate opioid receptors. Antagonists, on the other hand, still bind the same opioid receptors, but they activate them only mildly or even not at all, thus minimizing or preventing further effects.<ref name=":0" /> | ||
[[File:Syn bomb vacu 3.png|right|frameless|336x336px|Nerve Synapse]] | |||
* '''Strong Agonists:''' The main use of strong agonist opioids is in the treatment of severe pain. These opioids bond very strongly to receptors, primarily Mu (𝜇) receptors in the brain and spinal cord. Common strong agonist opioids include: Morphine, | * '''Strong Agonists:''' The main use of strong agonist opioids is in the treatment of severe pain. These opioids bond very strongly to receptors, primarily Mu (𝜇) receptors, in the brain and spinal cord. Common strong agonist opioids include: Morphine, Fentanyl, Meperidine, and Methadone. | ||
* '''Mild-to-Moderate Agonists:''' Mild-to-moderate agonists bond strongly to opioid receptors, but to a lesser degree than strong agonists. This results in reduced efficacy as well as effect of these agents. As such, this category of opioids is generally indicated for moderate pain. Examples include: Codeine, Hydrocodone, and Oxycodone. | * '''Mild-to-Moderate Agonists:''' Mild-to-moderate agonists bond strongly to opioid receptors, but to a lesser degree than strong agonists. This results in reduced efficacy as well as effect of these agents. As such, this category of opioids is generally indicated for moderate pain. Examples include: Codeine, Hydrocodone, and Oxycodone. | ||
* '''Mixed Agonist-Antagonist:''' | * '''Mixed Agonist-Antagonist:''' This category is defined by the phenomenon in which these agents act both as agonists (bind and activate) for certain opioid receptors, and antagonists (bind and don't activate) for other receptors. This characteristic can be useful in that these drugs can be used to achieve analgesia with fewer side effects. One example is the opioid butorphanol, which is an agonist for Kappa receptors and an antagonist for Mu receptors. The effect of this combination is the achievement of analgesia (activation of Kappa receptors) with a reduction in the risk of respiratory depression (binding with no activation of Mu receptors). Of particular note, while mixed agonist-antagonists generally carry less of a risk of dangerous side effects as well as dependency, they do carry a higher risk for psychotropic side effects such as hallucinations. | ||
* '''Antagonists:''' Pure antagonist opioids carry no analgesic properties due to their ability to bind receptors, but not activate them. As such, when antagonists bind opioid receptors, they block other opioids from | * '''Antagonists:''' Pure antagonist opioids carry no analgesic properties due to their ability to bind receptors, but not activate them. As such, when antagonists bind opioid receptors, they block other opioids from binding these same receptors. These characteristics make them ineffective in pain management, but highly effective at blocking or counteractive other opioids. This makes antagonists an important aspect of opioid management through their use in the treatment of addiction and overdose. The most common antagonist drug used in the USA is naloxone. | ||
== Benefits & Drawbacks of Opioids == | == Benefits & Drawbacks of Opioids == | ||
Opioids can produce profound analgesia for patients with acute pain. Opioids are very effective at treating | Opioids can produce profound analgesia for patients with acute and chronic pain. Opioids are very effective at treating pain due to their ability to inhibit pain signal transmission at multiple stages in the pain pathway, as well as their ability to enhance the action of inhibitory pain fibers. | ||
There are many adverse effects associated with opioids. Kappa receptor stimulation is associated with side effects such as hallucinations, dysphoria, anxiety and restlessness. Delta and mu receptor stimulation is often dangerously associated with respiratory depression | There are many adverse effects associated with opioids. Kappa receptor stimulation is associated with side effects such as hallucinations, dysphoria, anxiety, and restlessness. Delta and mu receptor stimulation is often dangerously associated with respiratory depression, making the body unable to regulate carbon dioxide levels effectively. Other drawbacks associated with opioid use in general include sleep apnea, hypothalami-pituitary axis suppression (hormonal changes with the potential for decreased libido, infertility & fluid retention), urinary retention, nausea, vomiting, physical dependence, addiction, opioid-induced hyperalgesia, dental pathology, constipation, increased mortality, and increased tolerance.<ref>Prescription Opioid Policy. A publication by The Royal Australian College of Physicians, Faculty of Pain Medicine ANZCA, The Royal Australian College of General Practitioners and The Royal Australian and New Zealand College of Psychiatrists.</ref> | ||
== Considerations in Physiotherapy == | == Considerations in Physiotherapy == | ||
Opioids are regularly administered for the treatment of pain; many patients that present to physical therapists may have already been prescribed them. | The prescription of opioids for the management of pain has increased significantly in the last few decades. A large contributor to this increase in the United States was the 2001 initiative by The Joint Commission, an independent group that oversees quality and practice within healthcare, to improve the management of patient's pain. As a result, pain quickly became known as the "fifth vital sign," sparking a dramatic increase in efforts to assess and manage pain. Unfortunately, this heightened emphasis on pain control also led to a dramatic uptick in the use of pain control medications, specifically opioids. Pharmaceutical companies began to highly promote these drugs after seeing an opportunity to provide a solution to a "newly recognized" problem. As opioid use increased, so did addiction and abuse of these medications. Between the years 2001 and 2013, opioid overdose deaths increased by at least 5 times in the United States.<ref name=":2">Mintken PE, Moore JR, Flynn TW. [https://www.jospt.org/doi/10.2519/jospt.2018.0606 Physical Therapists’ Role in Solving the Opioid Epidemic.] Journal of Orthopaedic & Sports Physical Therapy. 2018; 48(5): 349-353.</ref> | ||
Physical therapy can play a vital role in managing what is now considered by some to be an "opioid epidemic." Polling performed by Gallop indicated that in an American sample of 6200 adults, 78% would rather have their pain controlled by drug-free interventions compared to the use of opioids. While the actual follow-through is not this high, these figures highlight a desire by patients to have other options besides opioids for controlling pain, options that can be provided through physical therapy.<ref name=":2" /> | |||
A 2018 viewpoint article in the Journal of Orthopedic & Sports Physical Therapy suggested three ways that physical therapists can promote drug-free pain management among patients. The first area was education; physical therapists are in a unique position to provide information to both patients, and other practitioners, on the availability and effectiveness of therapy in managing both acute and chronic pain. Articles such as the 2018 JAMA study by Krebs, et al. clearly support physical therapy interventions as being at least as effective at improving pain related function in conditions such as knee osteoarthritis when compared with opioids. Education should also include helping patients to understand pain neuroscience, or the mechanisms of how pain occurs in the body. A 2016 study by Louw et al. showed that pain education (helping patients understand the physiology behind why they hurt) delivered to those with chronic pain can significantly reduce reported symptoms, improve function, and minimize healthcare utilization. The second area the viewpoint addressed was the need to promote early access to physical therapy. Multiple studies have shown that when physical therapy services are provided early in a patient's course of treatment, through aspects such as same-day access clinics or direct access care, a reduction is seen in metrics including cost, healthcare utilization, duration of recovery, and number of prescriptions for opioids. The final area addressed was prevention. Physical therapists, as practitioners, are specifically qualified to address a variety of aspects that contribute to poor health outcomes. Physical therapy interventions often include preventative screening, promotion of improved sleep and nutrition, increased physical activity, and many other aspects that can improve overall quality of life. As such, by addressing these topics, physical therapists can help patients manage or even prevent chronic pain.<ref name=":2" /> <ref>Louw A, Zimney K, Puentedura EJ, Diener I. [https://pubmed.ncbi.nlm.nih.gov/27351541/ The efficacy of pain neuroscience education on musculoskeletal pain: a systematic review of the literature.] Physiother Theory Pract. 2016; 32: 332– 355.</ref> <ref>Krebs EE, Gravely A, Nugent S, et al. [https://pubmed.ncbi.nlm.nih.gov/29509867/ Effect of opioid vs nonopioid medications on pain-related function in patients with chronic back pain or hip or knee osteoarthritis pain: the SPACE randomized clinical trial.] JAMA. 2018; 319: 872– 882.</ref> | |||
===== Physical Therapy and Patients Prescribed Opioids ===== | |||
Opioids are regularly administered for the treatment of pain; many patients that present to physical therapists may have already been prescribed them. This is even more common in those being treated for musculoskeletal pain, with opioids being prescribed in these cases as much as 30% of the time. In conjunction, as many as 75% of patients exhibiting opioid misuse behaviors report experiencing musculoskeletal pain. | |||
While prescription management does not generally fall within the scope of practice for physical therapists (the exception being limited privileges in areas of practice such as USA military providers), they can still play a vital role in screening for and referring patients who are at risk for opioid misuse. Below are several aspects that physical therapists should be aware of, and incorporate into their assessment and treatment of patients who complain of pain and/or have been prescribed opioids.<ref>Magel J, Kietrys D, Kruger ES, Fritz JM, Gordon AJ. [https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8576962/ Physical therapists should play a greater role in managing patients with opioid use and opioid misuse.] Subst Abus. 2021; 42(3): 255-260.</ref> | |||
* Inquire about any past or current use of prescription opioids | |||
* Do not judge a patient's use or need for opioids, but instead provide constructive guidance and education on safe use | |||
* Monitor for opioid misuse behaviors and discuss these with the patient | |||
* If opioid misuse is suspected, contact the referring physician (if the patient has one) or refer the patient to an addiction specialist | |||
* Maintain open and non-judgmental communication at all times, educating and guiding them when possible | |||
===== Recognizing Opioid Misuse ===== | |||
Identifying patients who may be experiencing opioid misuse can be as simple as being alert for certain behaviors, symptoms, or actions observed or voiced during a physical therapy session. Below are some common signs that, if noticed, may be reason for further inquiry with your patient.<ref>Mayo Clinic. How to tell is a loved one is abusing opioids. Available from: https://www.mayoclinic.org/diseases-conditions/prescription-drug-abuse/in-depth/how-to-tell-if-a-loved-one-is-abusing-opioids/art-20386038</ref><ref>Johns Hopkins Medicine. Opioid Use Disorder. Available from: https://www.hopkinsmedicine.org/health/conditions-and-diseases/opioid-use-disorder</ref> | |||
* Non-indicated use - taking more than a prescription calls for at a given time or taking the medication for the feeling it elicits | |||
* Preventative use - a patient may report using an opioid "just in case" or continued use when pain is not present | |||
* Changes in mood, mood patterns, or behaviors of isolation | |||
* Changes in sleep (sleeping more or less than usual) | |||
* Drowsiness, malaise, weight loss, or sexual changes | |||
* Altered routines or habits with no other apparent cause | |||
* "Borrowing" medication or "losing" a prescription to attain a refill | |||
* Redundant prescriptions - seeking additional or backup prescriptions from multiple doctors | |||
* Decreased inhibition, poor decision-making, reckless or dangerous actions or choices | |||
{{#ev:youtube|https://www.youtube.com/watch?v=Bp2lhI4ka4M|width}} | {{#ev:youtube|https://www.youtube.com/watch?v=Bp2lhI4ka4M|width}} | ||
Opioid use is also linked to increased [[falls]] | ===== Opioids use and risk of falls ===== | ||
{{#ev:youtube|https://www.youtube.com/watch?v=mYrQUj2yecs&app=desktop|width}}<ref>Fox 47 news. Opiod use linked to increased risk of falls. Available from: https://www.youtube.com/watch?v=mYrQUj2yecs&app=desktop (last accessed 9.4.2019)</ref> | Opioid use is also linked to increased risk of [[falls]]. A 2018 retrospective cohort study by Daoust et al. found an increased risk of falls, as well as higher incidence of death from fall-related injuries, in older adults who reported recent opioid use. Results also showed that opioid use was associated with increased length of stay in hospitals for treatment of these injuries. | ||
{{#ev:youtube|https://www.youtube.com/watch?v=mYrQUj2yecs&app=desktop|width}}<ref>Fox 47 news. Opiod use linked to increased risk of falls. Available from: https://www.youtube.com/watch?v=mYrQUj2yecs&app=desktop (last accessed 9.4.2019)</ref> | |||
== Links == | |||
[https://www.choosept.com/ American Physical Therapy Association: Choose PT Initiative] | |||
[https://www.cdc.gov/drugoverdose/index.html American Centers for Disease Control and Prevention (CDC): Drug Overdose Resources] | |||
[https://www.samhsa.gov/ American Substance Abuse and Mental Health Service Administration (SAMHSA)] | |||
[https://findtreatment.gov/ American SAMHSA Find Treatment Page] | |||
[ | [https://www.who.int/news-room/fact-sheets/detail/opioid-overdose World Health Organization: Opioid Overdose] | ||
== References == | == References == | ||
Latest revision as of 20:16, 22 February 2023
Introduction[edit | edit source]
An opioid (exogenous opioid) refers to any substance from a group of analgesic (pain relieving) agents derived from the substance opium. Opioids are a type of depressant, analgesic, drug that slows down the messages being sent through the central nervous system between the body and the brain. Although used to treat pain, opioids can entice euphoric feelings and sedative effects which can be addictive. These properties make abuse of this drug group increasingly common.
Opioids can be naturally occurring, synthetic, or semi-synthetic depending on how they are derived. Natural and semi-synthetic opioids utilize substances extracted from opium, which is produced from the dried seeds of the opium poppy. Fully synthetic opioids, on the other hand, are derived using laboratory methods to produce the same compounds naturally found in opium through controlled chemical processes. A least 20 different compounds are found in opium; these biologically active substances can either produce analgesic effects directly (i.e. morphine) or act as precursors that can be used to produce other analgesic drugs including semi-synthetic opioids.[1]
Clinical Pharmacology[edit | edit source]
Production of opioids begins with four naturally occurring alkaloids which can be isolated from the opium poppy seed (papaver somniferum). These plant-derived amines, which include morphine, codeine, papaverine, and thebaine, can then be used to produce many varieties of semi-synthetic opioids useful in clinical medicine. Common semi-synthetic opioids derived from these four substances include diamorphine, dihydrocodeine, buprenorphine, nalbuphine, naloxone, and oxycodone.[2]
Opioids exert their analgesic properties by binding to specialized "opioid" receptors that already exist on nerve cells at specific sites in the central and peripheral nervous systems. These receptors exist in concert with endogenous (produced by the body) opioid-like substances, both of which act as part of the body's natural process of controlling pain and inflammation. The three groups of endogenous opioid-like substances include endorphins, enkephalins, and dynorphins. In addition to pain control, endogenous opioid-like substances are believed to also play a role in other processes including response to stress, immune modulation, GI function, and eating.[1]
Opioid Receptors[edit | edit source]
There are three major types of opioid receptors, mu (𝜇), delta (𝛿), and kappa (𝜅), found throughout the body. Other opioid receptors do exist, i.e. nociceptin/orphanin FQ peptide receptors, however, no known exogenous opioid has been found to specifically act upon them. As such, opioids are commonly classified by their ability to exert an effect by binding to one of the three main receptors (𝜇, 𝛿, and 𝜅). All 3 receptors will produce analgesia when bound by an opioid, but the degree of effect is determined by how strongly a given opioid binds to a given receptor. This degree of "affinity" produces further sub-classifications under each receptor type.[1][2]
In addition to producing analgesia, each receptor type also produces a unique and distinct spectrum of additional, and often adverse, effects when bound and activated. This specificity is important when considering patient safety, especially in certain situations, or when pre-existing conditions could be exacerbated by the effects. Common unique effects of each receptor are listed below:[1]
- Mu (𝜇) - Sedative effects, depression of respiratory function, constipation, reduction in the release of neurotransmitters
- Delta (𝛿) - Sedative effects, constipation, psychoactive effects
- Kappa (𝜅) - Heightened release of hormones, reduction in the release of neurotransmitters
Opioid Mechanisms of Action[edit | edit source]
Opioids produce their analgesic effect by acting directly on the synapses of neurons in the brain, spinal cord, and peripheral tissues. Each of these three areas are unique in their characteristics and how opioids specifically exert their effect.[1]
- Spinal Cord: Opioid action at the level of the spinal cord occurs by inhibiting the transmission of pain signals traveling from peripheral nerves to the brain. This occurs when opioids bind to the presynaptic receptors of pain neurons in this area, resulting in a reduction in the release of pain-mediating neurotransmitters including Substance P.
- Brain: Opioid action in the brain occurs by altering the action of neurons responsible for the interpretation and transmission of pain signals. A common belief is that opioids facilitate increased activity of descending pain pathways by blocking the signals of interneurons that would typically inhibit these pathways. When this process occurs it is known as disinhibition. At the same time, increased descending pathway activity also blocks the transmission of ascending pain signals in the spinal cord through the same mechanism as opioids acting on this region.
- Peripheral Tissue: Opioid receptors have been found on peripheral sensory neurons, allowing opioids to act directly in these areas. When the receptors on these peripheral nerves are bound by opioids, the excitability of the neurons is reduced. In this way, analgesia is achieved by preventing pain signals from even being produced.
Categories of Opioid Drugs[edit | edit source]
Opioids are classified based on the characteristics of how they bind to opioid receptors in the body. Specifically, these classifications are heavily influenced by the strength, or affinity, to which the agent binds. Additionally, the specification of agonist versus antagonist is an important distinction. Agonist agents are defined by their ability to bind and activate opioid receptors. Antagonists, on the other hand, still bind the same opioid receptors, but they activate them only mildly or even not at all, thus minimizing or preventing further effects.[1]
- Strong Agonists: The main use of strong agonist opioids is in the treatment of severe pain. These opioids bond very strongly to receptors, primarily Mu (𝜇) receptors, in the brain and spinal cord. Common strong agonist opioids include: Morphine, Fentanyl, Meperidine, and Methadone.
- Mild-to-Moderate Agonists: Mild-to-moderate agonists bond strongly to opioid receptors, but to a lesser degree than strong agonists. This results in reduced efficacy as well as effect of these agents. As such, this category of opioids is generally indicated for moderate pain. Examples include: Codeine, Hydrocodone, and Oxycodone.
- Mixed Agonist-Antagonist: This category is defined by the phenomenon in which these agents act both as agonists (bind and activate) for certain opioid receptors, and antagonists (bind and don't activate) for other receptors. This characteristic can be useful in that these drugs can be used to achieve analgesia with fewer side effects. One example is the opioid butorphanol, which is an agonist for Kappa receptors and an antagonist for Mu receptors. The effect of this combination is the achievement of analgesia (activation of Kappa receptors) with a reduction in the risk of respiratory depression (binding with no activation of Mu receptors). Of particular note, while mixed agonist-antagonists generally carry less of a risk of dangerous side effects as well as dependency, they do carry a higher risk for psychotropic side effects such as hallucinations.
- Antagonists: Pure antagonist opioids carry no analgesic properties due to their ability to bind receptors, but not activate them. As such, when antagonists bind opioid receptors, they block other opioids from binding these same receptors. These characteristics make them ineffective in pain management, but highly effective at blocking or counteractive other opioids. This makes antagonists an important aspect of opioid management through their use in the treatment of addiction and overdose. The most common antagonist drug used in the USA is naloxone.
Benefits & Drawbacks of Opioids[edit | edit source]
Opioids can produce profound analgesia for patients with acute and chronic pain. Opioids are very effective at treating pain due to their ability to inhibit pain signal transmission at multiple stages in the pain pathway, as well as their ability to enhance the action of inhibitory pain fibers.
There are many adverse effects associated with opioids. Kappa receptor stimulation is associated with side effects such as hallucinations, dysphoria, anxiety, and restlessness. Delta and mu receptor stimulation is often dangerously associated with respiratory depression, making the body unable to regulate carbon dioxide levels effectively. Other drawbacks associated with opioid use in general include sleep apnea, hypothalami-pituitary axis suppression (hormonal changes with the potential for decreased libido, infertility & fluid retention), urinary retention, nausea, vomiting, physical dependence, addiction, opioid-induced hyperalgesia, dental pathology, constipation, increased mortality, and increased tolerance.[3]
Considerations in Physiotherapy[edit | edit source]
The prescription of opioids for the management of pain has increased significantly in the last few decades. A large contributor to this increase in the United States was the 2001 initiative by The Joint Commission, an independent group that oversees quality and practice within healthcare, to improve the management of patient's pain. As a result, pain quickly became known as the "fifth vital sign," sparking a dramatic increase in efforts to assess and manage pain. Unfortunately, this heightened emphasis on pain control also led to a dramatic uptick in the use of pain control medications, specifically opioids. Pharmaceutical companies began to highly promote these drugs after seeing an opportunity to provide a solution to a "newly recognized" problem. As opioid use increased, so did addiction and abuse of these medications. Between the years 2001 and 2013, opioid overdose deaths increased by at least 5 times in the United States.[4]
Physical therapy can play a vital role in managing what is now considered by some to be an "opioid epidemic." Polling performed by Gallop indicated that in an American sample of 6200 adults, 78% would rather have their pain controlled by drug-free interventions compared to the use of opioids. While the actual follow-through is not this high, these figures highlight a desire by patients to have other options besides opioids for controlling pain, options that can be provided through physical therapy.[4]
A 2018 viewpoint article in the Journal of Orthopedic & Sports Physical Therapy suggested three ways that physical therapists can promote drug-free pain management among patients. The first area was education; physical therapists are in a unique position to provide information to both patients, and other practitioners, on the availability and effectiveness of therapy in managing both acute and chronic pain. Articles such as the 2018 JAMA study by Krebs, et al. clearly support physical therapy interventions as being at least as effective at improving pain related function in conditions such as knee osteoarthritis when compared with opioids. Education should also include helping patients to understand pain neuroscience, or the mechanisms of how pain occurs in the body. A 2016 study by Louw et al. showed that pain education (helping patients understand the physiology behind why they hurt) delivered to those with chronic pain can significantly reduce reported symptoms, improve function, and minimize healthcare utilization. The second area the viewpoint addressed was the need to promote early access to physical therapy. Multiple studies have shown that when physical therapy services are provided early in a patient's course of treatment, through aspects such as same-day access clinics or direct access care, a reduction is seen in metrics including cost, healthcare utilization, duration of recovery, and number of prescriptions for opioids. The final area addressed was prevention. Physical therapists, as practitioners, are specifically qualified to address a variety of aspects that contribute to poor health outcomes. Physical therapy interventions often include preventative screening, promotion of improved sleep and nutrition, increased physical activity, and many other aspects that can improve overall quality of life. As such, by addressing these topics, physical therapists can help patients manage or even prevent chronic pain.[4] [5] [6]
Physical Therapy and Patients Prescribed Opioids[edit | edit source]
Opioids are regularly administered for the treatment of pain; many patients that present to physical therapists may have already been prescribed them. This is even more common in those being treated for musculoskeletal pain, with opioids being prescribed in these cases as much as 30% of the time. In conjunction, as many as 75% of patients exhibiting opioid misuse behaviors report experiencing musculoskeletal pain.
While prescription management does not generally fall within the scope of practice for physical therapists (the exception being limited privileges in areas of practice such as USA military providers), they can still play a vital role in screening for and referring patients who are at risk for opioid misuse. Below are several aspects that physical therapists should be aware of, and incorporate into their assessment and treatment of patients who complain of pain and/or have been prescribed opioids.[7]
- Inquire about any past or current use of prescription opioids
- Do not judge a patient's use or need for opioids, but instead provide constructive guidance and education on safe use
- Monitor for opioid misuse behaviors and discuss these with the patient
- If opioid misuse is suspected, contact the referring physician (if the patient has one) or refer the patient to an addiction specialist
- Maintain open and non-judgmental communication at all times, educating and guiding them when possible
Recognizing Opioid Misuse[edit | edit source]
Identifying patients who may be experiencing opioid misuse can be as simple as being alert for certain behaviors, symptoms, or actions observed or voiced during a physical therapy session. Below are some common signs that, if noticed, may be reason for further inquiry with your patient.[8][9]
- Non-indicated use - taking more than a prescription calls for at a given time or taking the medication for the feeling it elicits
- Preventative use - a patient may report using an opioid "just in case" or continued use when pain is not present
- Changes in mood, mood patterns, or behaviors of isolation
- Changes in sleep (sleeping more or less than usual)
- Drowsiness, malaise, weight loss, or sexual changes
- Altered routines or habits with no other apparent cause
- "Borrowing" medication or "losing" a prescription to attain a refill
- Redundant prescriptions - seeking additional or backup prescriptions from multiple doctors
- Decreased inhibition, poor decision-making, reckless or dangerous actions or choices
Opioids use and risk of falls[edit | edit source]
Opioid use is also linked to increased risk of falls. A 2018 retrospective cohort study by Daoust et al. found an increased risk of falls, as well as higher incidence of death from fall-related injuries, in older adults who reported recent opioid use. Results also showed that opioid use was associated with increased length of stay in hospitals for treatment of these injuries.
Links[edit | edit source]
American Physical Therapy Association: Choose PT Initiative
American Centers for Disease Control and Prevention (CDC): Drug Overdose Resources
American Substance Abuse and Mental Health Service Administration (SAMHSA)
American SAMHSA Find Treatment Page
World Health Organization: Opioid Overdose
References[edit | edit source]
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 Ciccone CD, editors. Pharmacology in Rehabilitation. 5th Ed. Philadelphia: F.A. Davis Company, 2016. p202-218.
- ↑ 2.0 2.1 Pathan, H., & Williams, J. (2012). Basic opioid pharmacology: an update. British journal of pain; 6(1): 11-6.
- ↑ Prescription Opioid Policy. A publication by The Royal Australian College of Physicians, Faculty of Pain Medicine ANZCA, The Royal Australian College of General Practitioners and The Royal Australian and New Zealand College of Psychiatrists.
- ↑ 4.0 4.1 4.2 Mintken PE, Moore JR, Flynn TW. Physical Therapists’ Role in Solving the Opioid Epidemic. Journal of Orthopaedic & Sports Physical Therapy. 2018; 48(5): 349-353.
- ↑ Louw A, Zimney K, Puentedura EJ, Diener I. The efficacy of pain neuroscience education on musculoskeletal pain: a systematic review of the literature. Physiother Theory Pract. 2016; 32: 332– 355.
- ↑ Krebs EE, Gravely A, Nugent S, et al. Effect of opioid vs nonopioid medications on pain-related function in patients with chronic back pain or hip or knee osteoarthritis pain: the SPACE randomized clinical trial. JAMA. 2018; 319: 872– 882.
- ↑ Magel J, Kietrys D, Kruger ES, Fritz JM, Gordon AJ. Physical therapists should play a greater role in managing patients with opioid use and opioid misuse. Subst Abus. 2021; 42(3): 255-260.
- ↑ Mayo Clinic. How to tell is a loved one is abusing opioids. Available from: https://www.mayoclinic.org/diseases-conditions/prescription-drug-abuse/in-depth/how-to-tell-if-a-loved-one-is-abusing-opioids/art-20386038
- ↑ Johns Hopkins Medicine. Opioid Use Disorder. Available from: https://www.hopkinsmedicine.org/health/conditions-and-diseases/opioid-use-disorder
- ↑ Fox 47 news. Opiod use linked to increased risk of falls. Available from: https://www.youtube.com/watch?v=mYrQUj2yecs&app=desktop (last accessed 9.4.2019)