The influence of antidepressant medication on physiologic processes and exercise
Introduction[edit | edit source]
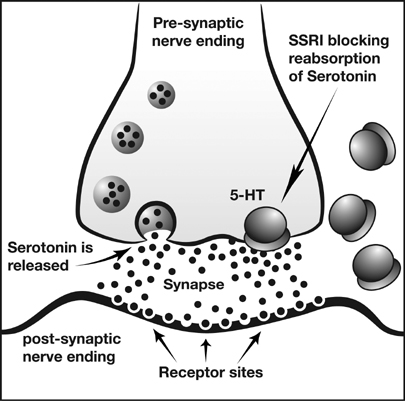
Antidepressants are used to treat, relieve, and prevent psychic depression. When used with exercise, different physiologic responses can occur. There are many types of antidepressant medications that can be effective at reducing depressive symptoms in patients. The main classifications this article will focus on are serotonin reuptake inhibitors (SSRIs), norepinephrine and dopamine reuptake inhibitors (NDRIs), tricyclic antidepressants (TCAs). TCAs were some of the initially marketed antidepressant medications in the 1950s and 1960s. [1] They are still prescribed to certain individuals, however they can often produce serious side effects and for the most part have been replaced by newer antidepressants. SSRIs and NDRIs are commonly prescribed antidepressant medications. They inhibit the reuptake of serotonin and norepinephrine/dopamine, respectively, allowing the neurotransmitters to stay in the synapse for a longer period of time. While these are some of the main types of antidepressant medications, some of the articles cited in the topics below may include other types of antidepressants as well.
Exercise Effects[edit | edit source]
The effects of antidepressants on individuals with high or low aerobic capacity can be assessed to evaluate the time to fatigue. Research was performed on paroxetine, a selective serotonin reuptake inhibitor, to determine its relationship to fatigue. Fatigue can be caused by a higher ratio of serotonin to dopamine in the brain, producing lethargy and lack of motivation. A specific dosage of 20 milligrams of paroxetine caused a decrease in total time of exercise until fatigue and performance in the individuals with higher aerobic capacity, which makes these individuals more responsive to the activation of serotonin than the low aerobic capacity group.[2] Therefore, antidepressant medication may be able to influence time to fatigue and exercise performance in individuals with high aerobic capacity. However, because of the variability of outcomes with dosage amounts, more reseach needs to be done before results can be accepted as conclusive.
Antidepressants can have effects on the functional outcomes and the recovery process. The effects are dependent on the type of anitdepressant consumed. Research has compared the effects of fluoxetine and maprotiline used with and without rehabilitation therapy on individuals who have experience a stroke to evaluate the effectiveness of the antidepressant medications. Fluoxetine, a drug that inhibits the reuptake of serotonin, increased good outcomes during the recovery process; whereas maprotiline, a norepinephrine reuptake inhibitor, caused the least amount of improvements.[3] While both medications lowered depression, only fluoxetine combined with rehabilitation therapy was more beneficial than rehabilitation alone. Maprotiline was less beneficial, which could have been due to its hinderance of recovery. Long-term treatment of fluoxetine could increase the spread of serotonin by blocking 5-HT sites to cause an increase in motor function and recovery.[3] Serotonergic antidepressants combined with rehabilitation therapy could assist in the recovery process and lead to better outcomes.
Cardiovascular System
[edit | edit source]
TCAs were the classic anti-depressant medication for decades before SSRIs were discovered. TCAs are now known to be potentially toxic to be cardiovascular system. With normal theraupeutic doses, hypotension is not uncommon. This is extremely relevant for physical therapy as hypotension can make a patient at-risk for falls and/or fractures. In cases of overdose, TCAs can cause fatal arrhythmias.[4]
While TCAs are still prescribed to certain individuals, they have mostly been replaced by SSRIs, NDRIs, and other antidepressant medications. These newer medications have proven to be just as effective in terms of relieving the symptoms of depression, but they have far fewer negative side effects than their earlier counterparts. Paroxetine is a commonly prescribed SSRI. A 1992 article from International Clinical Psychopharmacology discussed two randomized controlled trials (RCTs) concerning paroxetine. Both RCTs were double blind studies. The first study concerned the effects of paroxetine in patients diagnosed with clinical depression. The results showed that paroxetine did not significantly alter heart rate, blood pressure, or EKG variables in the experimental group. The second study compared paroxetine versus amitriptyline (a TCA) in healthy males. The results showed no effect on EKG, heart rate, or blood pressure in those who received paroxetine. Those who received amitriptyline showed increased heart rate (p<.05) and changes in EKG variables.[4]
Pulmonary System[edit | edit source]
Implications of popular anti-depressant medications, such as selective serotonin reuptake inhibitor (SSRI), include it side effects on the pulmonary system. Minimal research suggests that these medications may prevent or ameliorate pulmonary arterial hypertension (PAH). [5] A 2012 article studied the effects of SSRIs with the hypothesis that they might be useful for the prevention or treatment of PAH. This case-control study matched ten subjects with controls. The subjcets were individuals who developed PAH that required treatment with medication. The two groups were either exposed to SSRIs for non-SSRI antidepressant medication. The results of this study contradicted their hypothesis that SSRIs would prevent PAH, in fact a postive association was found between individuals who used SSRIs and the development of PAH. [5]
Metabolic System[edit | edit source]
The effects anti-depressant medications have on the metabolic system vary widely depending on the individual and the type of medication used. Some of the most common metabolic side effects are weight gain and weight loss. Research has shown that TCA’s and many SSRI’s share the common side effect of causing weight gain.[6][7][8][9] However, some SSRI’s have actually shown to have the exact opposite effect, depending on the specific SSRI used and on the individual.[10][11] NDRI’s have also shown to have the exact opposite effect, and have even been successfully used for the explicit purpose of increasing metabolism to lose weight. One specific NDRI drug that has become especially popular for its weight loss effects is Bupropion, widely known by the brand name Wellbutrin.[12][13][14] It is therefore of the utmost importance to note the type of anti-depressant medication patients are using when attempting to gain or lose weight through an exercises program
Neurological Effects[edit | edit source]
Conclusion[edit | edit source]
References[edit | edit source]
- ↑ Ramachandraih CT, Subramanyam N, Bar KJ, Baker G, &amp; Yeragani VK. Antidepressants: From MAOIs to SSRIs and more. Indian J Psychiatry. 2011;53(2):180–2. doi:10.4103/0019-5545.82567.
- ↑ Teixeira-Coelho, F, Uendeles-Pinto, J, Serafim, A, Wanner, S, Coelho, M, &amp;amp;amp;amp; Suares, D. The paroxetine effect on exercise performance depends on the aerobic capacity of exercising individuals. Journal of Sports Science and Medicine 2014; 13:232-243.
- ↑ 3.0 3.1 Dam, M, Tonin, P, De Boni, A, Pizzolato, G, Casson, S, Ermani, M, . . . Battistin, L. Effects of fluoxetine and maprotiline on functional recovery in poststroke hemiplegic patients undergoing rehabilitation therapy. Stroke 1996; 27: 1211-4.
- ↑ 4.0 4.1 Warrington SJ, Lewis Y. Cardiovascular effects of antidepressants: studies of paroxetine in healthy mean and depressed patients. Int Clin Psychopharmaco. 1992;6(4):59-64.
- ↑ 5.0 5.1 Dhalla IA, Jurrlick DN, Gomes T, Granton JT, Zheng H, Mamdani MM.Selective serotonin reuptake inhibitors and pulmonary arterial hypertension: a case control study. CHEST 2012;141:348-353. Full version: http://journal.publications.chestnet.org/data/Journals/CHEST/23327/110426.pdf (accessed 19 Nov 2015).
- ↑ Ciraulo, D. (2011). Clinical Pharmacology and Therapeutics of Antidepressants. In Pharmacotherapy of depression (2nd ed., pp. 53-56). New York: Humana Press.
- ↑ Ness-Abramof, R., Apovian, C.M., (2005). Drug-induced weight gain. Drugs Today, 41(8), 547-555. doi: 10.1358/dot.2005.41.8.893630
- ↑ Papakostas, G.I. (2008). Tolerability of modern antidepressants. Journal of Clinical Psychiatry, 69, 8-13.
- ↑ Masand, P.S., Gupta, S. (1999). Selective serotonin-reuptake inhibitors: an update. Harvard Review of Psychiatry, 7(2), 69-84.
- ↑ Li, Z., Maglione, M., Tu, W., Mojica, W., Arterburn, D., Shugarman, L.,R., . . . Morton, S.C. (2005). Meta-analysis: Pharmacologic treatment of obesity. Annals of Internal Medicine, 142(7), 532-546. doi:10.7326/0003-4819-142-7-200504050-00012
- ↑ Pijl, H., Meinders, A.E. (1996). Bodyweight change as an adverse effect of drug treatment. Mechanisms and management. Drug Safety, 14(5), 329-42.
- ↑ Gadde, K.M., Parker, C.B., Marner, L.G., Wagner, H.R., Logue, E.J., Drezner, M.K., Krishnan, K.R. (2001). Bupropion for weight loss: an investigation of efficacy and tolerability in overweight and obese women. Obesity Research, 9(9), 544-551.
- ↑ Jain, A., Kaplan, R., Gadde, K., Wadden, T., Allison, D., Brewer, E., . . . Metz, A. (2002). Bupropion SR vs. placebo for weight loss in obese patients with depressive symptoms. Obesity Research, 10(10), 1049-1056.
- ↑ Anderson, J., Greenway, F., Fujioka, K., Gadde, K., Mckenney, J., & O'neil, P. (2002). Bupropion SR enhances weight loss: A 48-week double-blind, placebo-controlled trial. Obesity Research, 10(7), 633-641.