Zellweger Syndrome
Original Editor - User Name
Top Contributors - Ayodeji Mark-Adewunmi
This article is currently under review and may not be up to date. Please come back soon to see the finished work!
Introduction[edit | edit source]
Zellweger Syndrome, also known as Zellweger Spectrum Disorders, is an uncommon inborn condition. It’s marked by a decrease or lack of functional peroxisomes in an individual’s cells. This syndrome is part of a group of disorders known as Zellweger spectrum disorders, which are classified as leukodystrophies. The syndrome is named after Hans Zellweger (1909–1990), a Swiss-American pediatrician who was a professor of pediatrics and genetics at the University of Iowa. He conducted extensive research on this disorder.
Cerebrohepatorenal syndrome, also known as Zellweger spectrum disorder, is a rare inherited condition characterized by the absence or reduction of functional peroxisomes in cells. These peroxisomes are crucial for the beta-oxidation of very long-chain fatty acids. The disorder is inherited in an autosomal recessive pattern, and it encompasses a range of disease phenotypes and severity levels, including Zellweger syndrome (ZS), neonatal adrenoleukodystrophy (NALD), infantile Refsum disease (IRD), and rhizomelic chondrodysplasia punctata type 1 (RCDP1).
Etiology[edit | edit source]
Zellweger syndrome is the result of a mutation in any of the 13 PEX genes. Most cases of Zellweger syndrome are due to a mutation in the PEX1 gene. These genes control peroxisomes, which are needed for normal cell function.
ZSDs are caused by mutations in one of the 13 different PEX genes. PEX genes encode proteins called peroxins and are involved in either peroxisome formation, peroxisomal protein import, or both. As a consequence, mutations in PEX genes cause a deficiency of functional peroxisomes.[1] Cells from ZSD patients either entirely lack functional peroxisomes, or cells can show a reduced number of functional peroxisomes or a mosaic pattern (i.e. a mixed population of cells with functional peroxisomes and cells without. Peroxisomes are involved in many anabolic and catabolic metabolic processes, like biosynthesis of ether phospholipids and bile acids, α- and β-oxidation of fatty acids and the detoxification of glyoxylate and reactive oxygen species. Dysfunctional peroxisomes therefore cause biochemical abnormalities in tissues,[2] but also in readily available materials like plasma and urine.[3]
Epidemiology[edit | edit source]
The incidence of ZSDs is estimated to be 1 in 50.000 newborns in the United States.[4] It is presumed that ZSDs occur worldwide, but the incidence may differ between regions. For example, the incidence of (classic) Zellweger syndrome in the French-Canadian region of Quebec was estimated to be 1 in 12. A much lower incidence is reported in Japan, with an estimated incidence of 1 in 500.000 births [19]. More accurate incidence data about ZSDs will become available in the near future, since newborn screening for X-linked adrenoleukodystrophy
Clinical Features[edit | edit source]
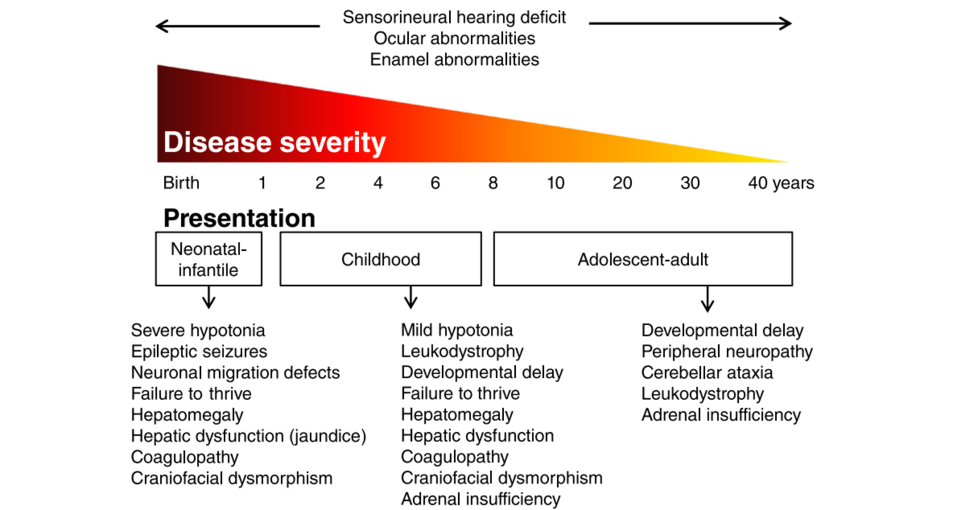
Patients with a ZSD can roughly be divided into three groups according to the age of presentation: the neonatal, infantile presentation, the childhood presentation and an adolescent-adult (late) presentation.
Neonatal-Infantile Presentation[edit | edit source]
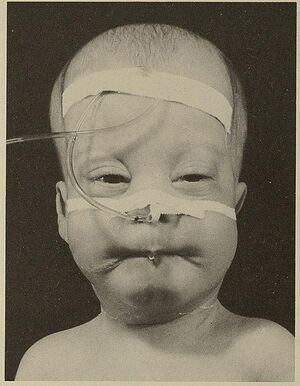
ZSD patients within this group typically present in the neonatal period with hepatic dysfunction and profound hypotonia resulting in prolonged jaundice and feeding difficulties. Epileptic seizures are usually present in these patients. Characteristic dysmorphic features can usually be found, of which the facial dysmorphic signs are most evident. Sensorineural deafness and ocular abnormalities like retinopathy, cataracts and glaucoma are typical but not always recognized at first presentation. Brain magnetic resonance imaging (MRI) may show neocortical dysplasia (especially perisylvian polymicrogyria), generalized decrease in white matter volume, delayed myelination, bilaterial ventricular dilatation and germinolytic cysts.[5] Neonatal onset leukodystrophy is rarely described.[6] Calcific stippling (chondrodysplasia punctata) may be present, especially in the knees and hips. The neonatal-infantile presentation grossly resembles what was originally described as classic ZS. Prognosis is poor and survival is usually not beyond the first year of life.
Childhood Presentation[edit | edit source]
These patients show a more varied symptomatology than ZSD patients with a neonatal-infantile presentation. Presentation usually involves delayed developmental milestone achievement. Ocular abnormalities comprise retinitis pigmentosa, cataract and glaucoma, often leading to early blindness and tunnel vision.[7]
Sensorineural deafness is almost always present and usually discovered by auditory screening programs. Hepatomegaly and hepatic dysfunction with coagulopathy, elevated transaminases and (history of ) hyperbilirubinemia are common. Some patients develop epileptic seizures. Craniofacial dysmorphic features are generally less pronounced than in the neonatal-infantile group.
Renal calcium oxalate stones and adrenal insufficiency may develop. Early-onset progressive leukodystrophy may occur, leading to loss of acquired skills and milestones in some individuals. The progressive demyelination is diffuse and affects the cerebrum, midbrain and cerebellum with involvement of the hilus of the dentate nucleus and the peridentate white matter.[8]
Sequential imaging in three ZSD patients showed that the earliest abnormalities related to demyelination were consistently seen in the hilus of the dentate nucleus and superior cerebellar peduncles, chronologically followed by the cerebellar white matter, brainstem tracts, parieto-occipital white matter, splenium of the corpus callosum and eventually involvement of the whole of the cerebral white matter.[9]
A small subgroup of patients develop a relatively late-onset white matter disease, but no patients with late-onset rapid progressive white matter disease after the age of five have been reported.[10] Prognosis depends on what organ systems are primarily affected (i.e. liver) and the occurrence of progressive cerebral demyelination, but life expectancy is decreased and most patients die before adolescence.
Adolescent-Adult Presentation[edit | edit source]
Symptoms in this group are less severe, and diagnosis can be in late child- or even adulthood.[11] Ocular abnormalities and a sensorineural hearing deficit are the most consistent symptoms. Craniofacial dysmorphic features can be present, but may also be completely absent.
Developmental delay is highly variable and some patients may have normal intelligence. Daily functioning ranges from completely independent to 24 h care. It is important to emphasize that primary adrenal insufficiency is common and is probably under diagnosed. In addition to some degree of developmental delay, other neurological abnormalities are usually also present:
- signs of peripheral neuropathy,
- cerebellar ataxia and
- pyramidal tract signs.
The clinical course is usually slowly progressive, although the disease may remain stable for many years. Slowly progressive, clinically silent leukoencephalopathy is common, but MRI may be normal in other cases.
Pathophysiology[edit | edit source]
Peroxisomes are single membrane-bounded organelles with a matrix containing over 50 enzymes for fatty acid metabolism. All human cells except erythrocytes contain peroxisomes. The liver and kidney have peroxisomes in abundance in comparison to other organs. Peroxins are necessary for the proper assembly of peroxisomes, and mutations in the peroxin gene (PEX) result in a defect in peroxisomal formation, which is associated with lower or undetectable levels of key internal enzymes. The peroxisomes are involved in beta-oxidation of very-long-chain fatty acids (VLCFA), alpha oxidation of branched-chain fatty acids, catabolism of amino acids and ethanol, biosynthesis of bile acids, steroid hormones, gluconeogenesis, and plasmalogen formation which are important constituents of the cell membrane and myelin. It is also involved in the degradation of cytotoxic hydrogen peroxide.[12]
Zellweger spectrum disorder is thus characterized by increased accumulation of VLCFA and increased C26 and C22 fatty acids in plasma, fibroblasts, and amniocytes.[13] Reduced steroid biosynthesis and accumulation of VLCFA in adrenal gland cells cause decreased levels of adrenocorticotropic hormone (ACTH) and some other steroidal hormones.[14] Reduced degradation of cytotoxic hydrogen peroxide and abnormal accumulation of VLCFA causes neuronal membrane injury and demyelination.[15]
Major abnormalities are present in the kidney (cortical cysts), liver (fibrotic), and brain (demyelination, centrosylvian polymicrogyria) - hence the name cerebrohepatorenal syndrome.
Evaluation[edit | edit source]
The initial diagnostic step is identifying clinical features and demonstrating elevated very-long-chain fatty acid (VLCFA) in blood during newborn screening. Genetic testing makes the diagnosis of PEX genes. The next step in evaluation is biochemical testing, looking for elevated levels of VLCFA, phytanic and/or pristanic acid, pipecolic acid, bile acid intermediates, and reduced levels of plasmalogen in red blood cells.[16] Patients with mild disease may have normal biochemical tests, so confirmation in cultured skin fibroblasts at 40 degrees centigrade is required if clinical suspicion is high.[17]Genetic counseling and prenatal diagnosis are crucial.[18]
Differential Diagnosis[edit | edit source]
Differential diagnosis of Zellweger spectrum disorder based on the main presenting symptom includes the following:
Hypotonia in newborns
- Chromosomal abnormalities (Down syndrome, Prader-Willi syndrome)
- Spinal muscular atrophy
- Hypoxic-ischemic encephalopathy
- Other peroxisomal disorders (acyl-CoA oxidase type 1 deficiency, D-bifunctional protein deficiency)[19]
Sensorineural hearing loss with retinitis pigmentosa
- Usher syndrome type 1,2
- Cockayne syndrome
- Alport syndrome
- Waardenburg syndrome
- Classical Refsum disease
Bilateral cataract
- Lowe syndrome
- Galactosemia
- Congenital infections
- Rhizomelic chondrodysplasia punctate
Adrenocortical Insufficiency
- Adrenal hemorrhage
- X-linked adrenoleukodystrophy
- Infectious adrenalitis
Prognosis[edit | edit source]
Children presenting in the neonatal period have a very poor prognosis and usually die within the first year of life. Patients who present in later childhood can develop progressive liver disease/failure and have slightly longer survival after diagnosis as compared to the neonatal form. Patients who present in adolescence have a slightly longer survival but usually develop progressive neurological symptoms, including spasticity and peripheral neuropathy, later in life.
Complications[edit | edit source]
- Gastrointestinal bleeding
- Liver failure
- Pneumonia
- Respiratory distress
- Infections
Medical Management[edit | edit source]
Zellweger spectrum disorder is a rapidly progressive disorder with a high mortality rate. With no curative treatment available, treatment options are limited to supportive care to improve quality of life.[20]
Various treatment modalities that have been tried include:
1. Docosahexaenoic acid - This is a long-chain unsaturated fatty acid essential for myelination, brain, and eye development. The levels of docosahexaenoic acid are low in the plasma of patients with ZS. However, its replacement was not associated with improved neurological symptoms or visual disturbances in randomized controlled trials.[21]
2. Lorenzo's oil - Lorenzo's oil is a mixture of glyceryl trioleate and glyceryl trierucate, and its use was initially attempted in patients with X-linked adrenoleukodystrophy. It was shown to reduce VLCFA levels in plasma but did not affect disease progression in patients.[22][23]
3. Cholic acid - This is a 24-carbon bile acid, which is helpful in the absorption of fat-soluble vitamins. Due to liver dysfunction and lipoprotein synthesis impairment in patients with Zellweger spectrum disorder, there is a deficiency of fat-soluble vitamins, and the use of cholic acid has been tried in other hepatic function disorders. The US FDA has approved it for use in patients. However, there is little evidence regarding its efficacy.[24]
Supportive measures include:
- Hearing aids or cochlear implants for hearing loss
- Ophthalmologist referral, cataract removal, and glasses for vision impairment
- Standard antiepileptic drugs for seizures
- Vitamin K supplementation for coagulopathy
- Cortisone for adrenal insufficiency
- Gastrostomy for insufficient calorie intake
- Vitamin supplementation for low levels of fat-soluble vitamins (A, D, E)
References[edit | edit source]
- ↑ Waterham HR, Ebberink MS. Genetics and molecular basis of human peroxisome biogenesis disorders. Biochimica et Biophysica Acta (BBA)-Molecular Basis of Disease. 2012 Sep 1;1822(9):1430-41.
- ↑ Braverman NE, D'Agostino MD, MacLean GE. Peroxisome biogenesis disorders: Biological, clinical and pathophysiological perspectives. Developmental disabilities research reviews. 2013 Jun;17(3):187-96.
- ↑ Wanders RJ, Waterham HR. Peroxisomal disorders I: biochemistry and genetics of peroxisome biogenesis disorders. Clinical genetics. 2005 Feb;67(2):107-33.
- ↑ Steinberg SJ, Dodt G, Raymond GV, Braverman NE, Moser AB, Moser HW. Peroxisome biogenesis disorders. Biochimica et Biophysica Acta (BBA)-Molecular Cell Research. 2006 Dec 1;1763(12):1733-48.
- ↑ Poll-The BT, Gärtner J. Clinical diagnosis, biochemical findings and MRI spectrum of peroxisomal disorders. Biochimica et Biophysica Acta (BBA)-Molecular Basis of Disease. 2012 Sep 1;1822(9):1421-9.
- ↑ Poll‐The BT, Gootjes J, Duran M, De Klerk JB, Maillette de Buy Wenniger‐Prick LJ, Admiraal RJ, Waterham HR, Wanders RJ, Barth PG. Peroxisome biogenesis disorders with prolonged survival: phenotypic expression in a cohort of 31 patients. American Journal of Medical Genetics Part A. 2004 May 1;126(4):333-8.
- ↑ Hamel C. Retinitis pigmentosa. Orphanet journal of rare diseases. 2006 Dec;1(1):1-2.
- ↑ Poll-The BT, Gärtner J. Clinical diagnosis, biochemical findings and MRI spectrum of peroxisomal disorders. Biochimica et Biophysica Acta (BBA)-Molecular Basis of Disease. 2012 Sep 1;1822(9):1421-9.
- ↑ Van der Knaap MS, Wassmer E, Wolf NI, Ferreira P, Topçu M, Wanders RJ, Waterham HR, Ferdinandusse S. MRI as diagnostic tool in early-onset peroxisomal disorders. Neurology. 2012 Apr 24;78(17):1304-8.
- ↑ Barth PG, Gootjes J, Bode H, Vreken P, Majoie CB, Wanders RJ. Late onset white matter disease in peroxisome biogenesis disorder. Neurology. 2001 Dec 11;57(11):1949-55.
- ↑ Moser AB, Rasmussen M, Naidu S, Watkins PA, McGuinness M, Hajra AK, Chen G, Raymond G, Liu A, Gordon D, Garnaas K. Phenotype of patients with peroxisomal disorders subdivided into sixteen complementation groups. The Journal of pediatrics. 1995 Jul 1;127(1):13-22.
- ↑ Roth KS. Peroxisomal disease-common ground for pediatrician, cell biologist, biochemist, pathologist, and neurologist. Clinical pediatrics. 1999 Mar;38(2):73-5.
- ↑ Moser AB, Kreiter N, Bezman L, Lu SE, Raymond GV, Naidu S, Moser HW. Plasma very long chain fatty acids in 3,000 peroxisome disease patients and 29,000 controls. Annals of Neurology: Official Journal of the American Neurological Association and the Child Neurology Society. 1999 Jan;45(1):100-10.
- ↑ Knazek RA, Rizzo WB, Schulman JD, Dave JR. Membrane microviscosity is increased in the erythrocytes of patients with adrenoleukodystrophy and adrenomyeloneuropathy. The Journal of Clinical Investigation. 1983 Jul 1;72(1):245-8.
- ↑ Powers JM, Moser HW. Peroxisomal disorders: genotype, phenotype, major neuropathologic lesions, and pathogenesis. Brain Pathology. 1998 Jan;8(1):101-20.
- ↑ Braverman NE, Raymond GV, Rizzo WB, Moser AB, Wilkinson ME, Stone EM, Steinberg SJ, Wangler MF, Rush ET, Hacia JG, Bose M. Peroxisome biogenesis disorders in the Zellweger spectrum: An overview of current diagnosis, clinical manifestations, and treatment guidelines. Molecular genetics and metabolism. 2016 Mar 1;117(3):313-21.
- ↑ Klouwer FC, Berendse K, Ferdinandusse S, Wanders RJ, Engelen M, Poll-The BT. Zellweger spectrum disorders: clinical overview and management approach. Orphanet journal of rare diseases. 2015 Dec;10:1-1.
- ↑ Rafique M, Zia S, Rana MN, Mostafa OA. Zellweger syndrome—a lethal peroxisome biogenesis disorder. Journal of Pediatric Endocrinology and Metabolism. 2013 Apr 1;26(3-4):377-9.
- ↑ Klouwer FC, Berendse K, Ferdinandusse S, Wanders RJ, Engelen M, Poll-The BT. Zellweger spectrum disorders: clinical overview and management approach. Orphanet journal of rare diseases. 2015 Dec;10:1-1.
- ↑ Kheir AE. Zellweger syndrome: A cause of neonatal hypotonia and seizures. Sudanese Journal of Paediatrics. 2011;11(2):54.
- ↑ Paker AM, Sunness JS, Brereton NH, Speedie LJ, Albanna L, Dharmaraj S, Moser AB, Jones RO, Raymond GV. Docosahexaenoic acid therapy in peroxisomal diseases: results of a double-blind, randomized trial. Neurology. 2010 Aug 31;75(9):826-30.
- ↑ Aubourg P, Adamsbaum C, Lavallard-Rousseau MC, Rocchiccioli F, Cartier N, Jambaque I, Jakobezak C, Lemaitre A, Boureau F, Wolf C, Bougneres PF. A two-year trial of oleic and erucic acids (“Lorenzo's oil”) as treatment for adrenomyeloneuropathy. New England Journal of Medicine. 1993 Sep 9;329(11):745-52.
- ↑ Arai Y, Kitamura Y, Hayashi M, Oshida K, Shimizu T, Yamashiro Y. Effect of dietary Lorenzo's oil and docosahexaenoic acid treatment for Zellweger syndrome. Congenital anomalies. 2008 Dec;48(4):180-2.
- ↑ Keane MH, Overmars H, Wikander TM, Ferdinandusse S, Duran M, Wanders RJ, Faust PL. Bile acid treatment alters hepatic disease and bile acid transport in peroxisome‐deficient PEX2 Zellweger mice. Hepatology. 2007 Apr;45(4):982-97.