Zellweger Syndrome
Original Editor - User Name
Top Contributors - Ayodeji Mark-Adewunmi
This article is currently under review and may not be up to date. Please come back soon to see the finished work!
Introduction[edit | edit source]
Zellweger Syndrome, also known as Zellweger Spectrum Disorders, is an uncommon inborn condition. It’s marked by a decrease or lack of functional peroxisomes in an individual’s cells.[1] This syndrome is part of a group of disorders known as Zellweger spectrum disorders, which are classified as leukodystrophies. The syndrome is named after Hans Zellweger (1909–1990), a Swiss-American pediatrician who was a professor of pediatrics and genetics at the University of Iowa. He conducted extensive research on this disorder.
Zellweger Spectrum Disorder, also called cerebrohepatorenal syndrome, is a genetic condition that is very rare. It is caused by the absence or reduction of functional peroxisomes in cells. These peroxisomes are crucial for the beta-oxidation of very long-chain fatty acids. The disorder is inherited in an autosomal recessive pattern, and it encompasses a range of disease phenotypes and severity levels,[2] including Zellweger syndrome (ZS), neonatal adrenoleukodystrophy (NALD), infantile Refsum disease (IRD), and rhizomelic chondrodysplasia punctata type 1 (RCDP1).
Etiology[edit | edit source]
Zellweger syndrome is a genetic disorder that is caused by mutations in any of the 13 PEX genes. The PEX genes provide instructions for making peroxisomes, which are essential for normal cell function. Most cases of Zellweger syndrome are due to a mutation in the PEX1 gene.
A deficiency of functional peroxisomes is caused by mutations in one of the 13 different PEX genes, resulting in Zellweger Spectrum Disorders (ZSDs). These PEX genes encode peroxins, which are proteins involved in either the formation of peroxisomes, the import of peroxisomal proteins, or both. Hence, mutations in PEX genes lead to an inadequacy of functional peroxisomes.[1]
In Zellweger Spectrum Disorder (ZSD) patients, peroxisomes are either completely absent or present in reduced numbers, or in a mosaic pattern - meaning a mixed population of cells with functional peroxisomes and cells without. Peroxisomes play a crucial role in various metabolic processes, including anabolic and catabolic pathways such as the biosynthesis of ether phospholipids and bile acids, α- and β-oxidation of fatty acids, and the detoxification of glyoxylate and reactive oxygen species.
Due to the peroxisomes' dysfunctionality, there are biochemical abnormalities in tissues,[2] as well as readily accessible materials such as urine and plasma.[3]
Epidemiology[edit | edit source]
The estimated incidence of Zellweger Spectrum Disorders (ZSDs) in the United States is 1 in 50,000 newborns.[4] It is presumed that ZSDs occur globally, but the incidence may vary among regions. For instance, the estimated incidence of (classic) Zellweger syndrome in the French-Canadian region of Quebec was 1 in 12, while a significantly lower incidence was reported in Japan, with an estimated incidence of 1 in 500,000 births. With newborn screening for X-linked adrenoleukodystrophy, more precise incidence data about ZSDs will soon become available.
Clinical Features[edit | edit source]
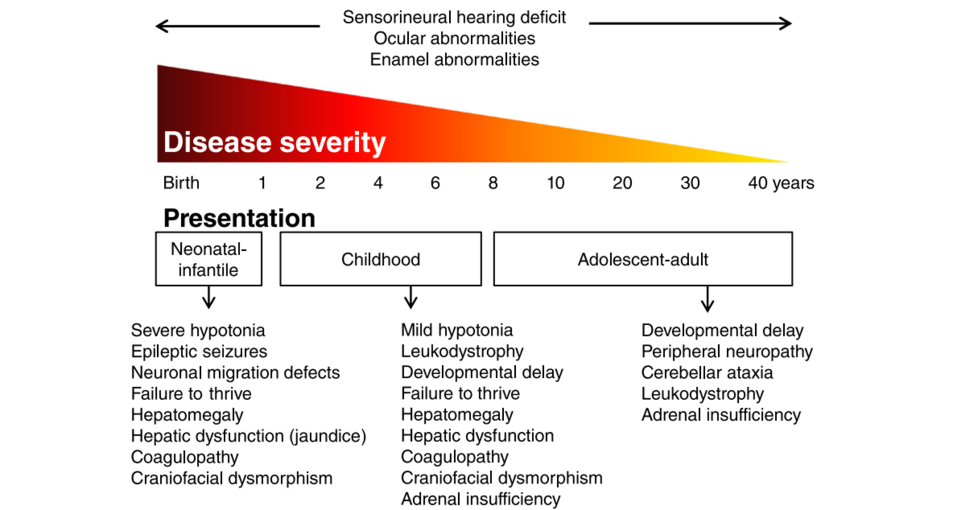
Zellweger Spectrum Disorder (ZSD) patients can be classified into three groups based on the age of onset: neonatal, infantile, and childhood presentation, as well as an adolescent-adult (late) presentation.
Neonatal-Infantile Presentation[edit | edit source]
In this group, Zellweger Spectrum Disorder (ZSD) patients typically exhibit hepatic dysfunction, profound hypotonia, prolonged jaundice, and feeding difficulties during the neonatal period. Epileptic seizures are frequently observed in these patients, along with characteristic dysmorphic features, with facial dysmorphic signs being the most prominent. Even though it is not always recognized during the initial presentation, sensorineural deafness and ocular abnormalities such as retinopathy, cataracts, and glaucoma are typical of this group. Brain magnetic resonance imaging (MRI) might show neocortical dysplasia (especially perisylvian polymicrogyria), bilaterial ventricular dilatation, delayed myelination, generalized decrease in white matter volume, and germinolytic cysts.[5] Neonatal onset leukodystrophy is rarely described.[6]
Calcific stippling, also known as chondrodysplasia punctata, can often be observed, particularly in the hip and knee regions. The condition in its neonatal-infantile form bears a striking resemblance to what is typically referred to as classic Zellweger Syndrome (ZS). Unfortunately, the outlook for this condition is not optimistic, with most individuals not surviving past their first year of life.
Childhood Presentation[edit | edit source]
Patients with this condition tend to exhibit a broader range of symptoms compared to those with a neonatal-infantile presentation of Zellweger Spectrum Disorder (ZSD). The symptoms often include a delay in reaching developmental milestones. Eye-related complications such as retinitis pigmentosa, cataracts, and glaucoma are common, frequently resulting in early onset blindness and restricted field of vision, also known as tunnel vision.[7]
Sensorineural deafness is almost always present and typically identified through auditory screening programs. Hepatic dysfunction with coagulopathy, elevated transaminases, and a history of hyperbilirubinemia are common, along with hepatomegaly. Some patients also experience epileptic seizures. Craniofacial dysmorphic signs are generally less prominent than in the neonatal-infantile group.
Individuals with Zellweger Spectrum Disorder may develop renal calcium oxalate stones and adrenal insufficiency. Some may experience early-onset progressive leukodystrophy, which can cause the loss of acquired skills and milestones. The demyelination is diffuse and progressive, primarily affecting the cerebrum, midbrain, and cerebellum, with associated involvement of the hilus of the dentate nucleus and the peridentate white matter..[8]
In three Zellweger Spectrum Disorder patients, sequential imaging revealed that the initial signs of demyelination were consistently observed in the hilus of the dentate nucleus and superior cerebellar peduncles, followed in chronological order by the cerebellar white matter, brainstem tracts, parieto-occipital white matter, splenium of the corpus callosum, and eventually, the entire cerebral white matter was affected.[9]
A minor subset of patients experience a relatively late onset of white matter disease. However, there have been no reported cases of patients developing a rapidly progressing white matter disease after reaching the age of five..[10] The outlook for patients largely depends on which organ systems are most affected, such as the liver, and whether they experience progressive cerebral demyelination. However, life expectancy is generally reduced, with most patients not surviving past their adolescent years.
Adolescent-Adult Presentation[edit | edit source]
Symptoms in this group are less severe, and diagnosis can be in late child- or even adulthood.[11] The most consistent symptoms of Zellweger Spectrum Disorder are sensorineural hearing deficit and ocular abnormalities. While craniofacial dysmorphic features may be present, they may also be completely absent.
The degree of developmental delay in patients with Zellweger Spectrum Disorder is highly variable, and some individuals may have normal intelligence. Daily functioning can range from complete independence to requiring 24-hour care. Primary adrenal insufficiency is common and likely underdiagnosed, making it essential to emphasize its presence. Other neurological abnormalities are typically present in addition to some level of developmental delay;
- signs of peripheral neuropathy,
- cerebellar ataxia and
- pyramidal tract signs.
The disease typically follows a slow progression, although it can remain stable for an extended period. A slow but progressive, clinically silent white matter disease in the brain, known as leukoencephalopathy, is common. However, in some cases, the magnetic resonance imaging (MRI) results may appear normal.
Pathophysiology[edit | edit source]
Peroxisomes are organelles with a single membrane, containing over 50 enzymes for fatty acid metabolism. All human cells, except erythrocytes, contain peroxisomes, with the liver and kidney having a greater abundance when compared to other organs. Proper peroxisomal assembly requires peroxins, and mutations in the peroxin gene (PEX) result in a defect in peroxisomal formation, causing lower or undetectable levels of key internal enzymes. Peroxisomes are involved in various metabolic processes, such as beta-oxidation of very-long-chain fatty acids (VLCFA), alpha oxidation of branched-chain fatty acids, catabolism of amino acids and ethanol, biosynthesis of bile acids, steroid hormones, gluconeogenesis, and plasmalogen formation, which are essential constituents of the cell membrane and myelin. Additionally, peroxisomes play a role in the degradation of cytotoxic hydrogen peroxide.[12]
Zellweger spectrum disorder is thus characterized by increased accumulation of VLCFA and increased C26 and C22 fatty acids in plasma, fibroblasts, and amniocytes.[13] Reduced steroid biosynthesis and accumulation of VLCFA in adrenal gland cells cause decreased levels of adrenocorticotropic hormone (ACTH) and some other steroidal hormones.[14] Reduced degradation of cytotoxic hydrogen peroxide and abnormal accumulation of VLCFA causes neuronal membrane injury and demyelination.[15]
Major abnormalities are present in the kidney (cortical cysts), liver (fibrotic), and brain (demyelination, centrosylvian polymicrogyria) - hence the name cerebrohepatorenal syndrome.
Evaluation[edit | edit source]
The first step in diagnosing this condition involves recognizing the clinical symptoms and detecting elevated levels of very-long-chain fatty acids (VLCFA) in the blood during newborn screening. The diagnosis of PEX genes is confirmed through genetic testing. Following this, biochemical testing is performed to look for increased levels of VLCFA, phytanic and/or pristanic acid, pipecolic acid, bile acid intermediates, and decreased levels of plasmalogen in red blood cells.[16]
In patients with a milder form of the disease, biochemical tests may yield normal results. Therefore, if there is a high clinical suspicion, confirmation is required through testing of cultured skin fibroblasts at a temperature of 40 degrees Celsius..[17]Genetic counseling and prenatal diagnosis are crucial.[18]
Differential Diagnosis[edit | edit source]
Differential diagnosis of Zellweger spectrum disorder based on the main presenting symptom includes the following:
Hypotonia in newborns
- Chromosomal abnormalities (Down syndrome, Prader-Willi syndrome)
- Spinal muscular atrophy
- Hypoxic-ischemic encephalopathy
- Other peroxisomal disorders (acyl-CoA oxidase type 1 deficiency, D-bifunctional protein deficiency)[19]
Sensorineural hearing loss with retinitis pigmentosa
- Usher syndrome type 1,2
- Cockayne syndrome
- Alport syndrome
- Waardenburg syndrome
- Classical Refsum disease
Bilateral cataract
- Lowe syndrome
- Galactosemia
- Congenital infections
- Rhizomelic chondrodysplasia punctate
Adrenocortical Insufficiency
- Adrenal hemorrhage
- X-linked adrenoleukodystrophy
- Infectious adrenalitis
Prognosis[edit | edit source]
Children who show symptoms in the neonatal period typically have a very grim outlook and often do not survive past their first year. Those who start showing symptoms later in their childhood may develop a progressive liver disease or liver failure, and generally have a slightly longer lifespan compared to those with the neonatal form. Adolescents who present with the disease also tend to live slightly longer, but they usually experience progressive neurological symptoms, including muscle stiffness (spasticity) and damage to the peripheral nerves (peripheral neuropathy), as they age.
Complications[edit | edit source]
- Gastrointestinal bleeding
- Liver failure
- Pneumonia
- Respiratory distress
- Infections
Medical Management[edit | edit source]
Zellweger Spectrum Disorder is an aggressive disorder that progresses quickly and has a high mortality rate. Unfortunately, there is no known cure for this condition, so treatment options are limited to supportive care. The focus is on improving the quality of life for those affected by the disorder.[20]
Various treatment modalities that have been tried include:
1. Docosahexaenoic acid - It's a long-chain unsaturated fatty acid that plays a crucial role in the development of myelin, brain, and eyes. Unfortunately, patients with Zellweger Spectrum Disorder have low levels of this essential fatty acid in their plasma. Despite this, replacing docosahexaenoic acid has not been shown to improve neurological symptoms or visual disturbances in randomized controlled trials.[21]
2. Lorenzo's oil - Lorenzo's oil is a combination of glyceryl trioleate and glyceryl trierucate. Initially, it was tried as a treatment for patients with X-linked adrenoleukodystrophy. While it did reduce VLCFA levels in plasma, it was not found to have any effect on the progression of the disease in patients.[22][23]
3. Cholic acid - Cholic acid is a bile acid that consists of 24 carbons and plays a vital role in the absorption of vitamins that are fat-soluble. In patients with Zellweger Spectrum Disorder, the dysfunction of their liver and impairment of lipoprotein synthesis result in a deficiency of fat-soluble vitamins. As a result, cholic acid has been used to address the issue in other hepatic function disorders. It has been approved by the United States FDA for use in patients, but there is limited evidence available regarding its effectiveness.[24]
Supportive measures include:
- Hearing aids or cochlear implants for hearing loss
- Ophthalmologist referral, cataract removal, and glasses for vision impairment
- Standard antiepileptic drugs for seizures
- Vitamin K supplementation for coagulopathy
- Cortisone for adrenal insufficiency
- Gastrostomy for insufficient calorie intake
- Vitamin supplementation for low levels of fat-soluble vitamins (A, D, E)
Physiotherapy Management[edit | edit source]
References[edit | edit source]
- ↑ 1.0 1.1 Waterham HR, Ebberink MS. Genetics and molecular basis of human peroxisome biogenesis disorders. Biochimica et Biophysica Acta (BBA)-Molecular Basis of Disease. 2012 Sep 1;1822(9):1430-41.
- ↑ 2.0 2.1 Braverman NE, D'Agostino MD, MacLean GE. Peroxisome biogenesis disorders: Biological, clinical and pathophysiological perspectives. Developmental disabilities research reviews. 2013 Jun;17(3):187-96.
- ↑ Wanders RJ, Waterham HR. Peroxisomal disorders I: biochemistry and genetics of peroxisome biogenesis disorders. Clinical genetics. 2005 Feb;67(2):107-33.
- ↑ Waterham HR, Ebberink MS. Genetics and molecular basis of human peroxisome biogenesis disorders. Biochimica et Biophysica Acta (BBA)-Molecular Basis of Disease. 2012 Sep 1;1822(9):1430-41.
- ↑ Poll-The BT, Gärtner J. Clinical diagnosis, biochemical findings and MRI spectrum of peroxisomal disorders. Biochimica et Biophysica Acta (BBA)-Molecular Basis of Disease. 2012 Sep 1;1822(9):1421-9.
- ↑ Poll‐The BT, Gootjes J, Duran M, De Klerk JB, Maillette de Buy Wenniger‐Prick LJ, Admiraal RJ, Waterham HR, Wanders RJ, Barth PG. Peroxisome biogenesis disorders with prolonged survival: phenotypic expression in a cohort of 31 patients. American Journal of Medical Genetics Part A. 2004 May 1;126(4):333-8.
- ↑ Hamel C. Retinitis pigmentosa. Orphanet journal of rare diseases. 2006 Dec;1(1):1-2.
- ↑ Poll-The BT, Gärtner J. Clinical diagnosis, biochemical findings and MRI spectrum of peroxisomal disorders. Biochimica et Biophysica Acta (BBA)-Molecular Basis of Disease. 2012 Sep 1;1822(9):1421-9.
- ↑ Van der Knaap MS, Wassmer E, Wolf NI, Ferreira P, Topçu M, Wanders RJ, Waterham HR, Ferdinandusse S. MRI as diagnostic tool in early-onset peroxisomal disorders. Neurology. 2012 Apr 24;78(17):1304-8.
- ↑ Barth PG, Gootjes J, Bode H, Vreken P, Majoie CB, Wanders RJ. Late onset white matter disease in peroxisome biogenesis disorder. Neurology. 2001 Dec 11;57(11):1949-55.
- ↑ Moser AB, Rasmussen M, Naidu S, Watkins PA, McGuinness M, Hajra AK, Chen G, Raymond G, Liu A, Gordon D, Garnaas K. Phenotype of patients with peroxisomal disorders subdivided into sixteen complementation groups. The Journal of pediatrics. 1995 Jul 1;127(1):13-22.
- ↑ Roth KS. Peroxisomal disease-common ground for pediatrician, cell biologist, biochemist, pathologist, and neurologist. Clinical pediatrics. 1999 Mar;38(2):73-5.
- ↑ Moser AB, Kreiter N, Bezman L, Lu SE, Raymond GV, Naidu S, Moser HW. Plasma very long chain fatty acids in 3,000 peroxisome disease patients and 29,000 controls. Annals of Neurology: Official Journal of the American Neurological Association and the Child Neurology Society. 1999 Jan;45(1):100-10.
- ↑ Knazek RA, Rizzo WB, Schulman JD, Dave JR. Membrane microviscosity is increased in the erythrocytes of patients with adrenoleukodystrophy and adrenomyeloneuropathy. The Journal of Clinical Investigation. 1983 Jul 1;72(1):245-8.
- ↑ Powers JM, Moser HW. Peroxisomal disorders: genotype, phenotype, major neuropathologic lesions, and pathogenesis. Brain Pathology. 1998 Jan;8(1):101-20.
- ↑ Braverman NE, Raymond GV, Rizzo WB, Moser AB, Wilkinson ME, Stone EM, Steinberg SJ, Wangler MF, Rush ET, Hacia JG, Bose M. Peroxisome biogenesis disorders in the Zellweger spectrum: An overview of current diagnosis, clinical manifestations, and treatment guidelines. Molecular genetics and metabolism. 2016 Mar 1;117(3):313-21.
- ↑ Klouwer FC, Berendse K, Ferdinandusse S, Wanders RJ, Engelen M, Poll-The BT. Zellweger spectrum disorders: clinical overview and management approach. Orphanet journal of rare diseases. 2015 Dec;10:1-1.
- ↑ Rafique M, Zia S, Rana MN, Mostafa OA. Zellweger syndrome—a lethal peroxisome biogenesis disorder. Journal of Pediatric Endocrinology and Metabolism. 2013 Apr 1;26(3-4):377-9.
- ↑ Klouwer FC, Berendse K, Ferdinandusse S, Wanders RJ, Engelen M, Poll-The BT. Zellweger spectrum disorders: clinical overview and management approach. Orphanet journal of rare diseases. 2015 Dec;10:1-1.
- ↑ Kheir AE. Zellweger syndrome: A cause of neonatal hypotonia and seizures. Sudanese Journal of Paediatrics. 2011;11(2):54.
- ↑ Paker AM, Sunness JS, Brereton NH, Speedie LJ, Albanna L, Dharmaraj S, Moser AB, Jones RO, Raymond GV. Docosahexaenoic acid therapy in peroxisomal diseases: results of a double-blind, randomized trial. Neurology. 2010 Aug 31;75(9):826-30.
- ↑ Aubourg P, Adamsbaum C, Lavallard-Rousseau MC, Rocchiccioli F, Cartier N, Jambaque I, Jakobezak C, Lemaitre A, Boureau F, Wolf C, Bougneres PF. A two-year trial of oleic and erucic acids (“Lorenzo's oil”) as treatment for adrenomyeloneuropathy. New England Journal of Medicine. 1993 Sep 9;329(11):745-52.
- ↑ Arai Y, Kitamura Y, Hayashi M, Oshida K, Shimizu T, Yamashiro Y. Effect of dietary Lorenzo's oil and docosahexaenoic acid treatment for Zellweger syndrome. Congenital anomalies. 2008 Dec;48(4):180-2.
- ↑ Keane MH, Overmars H, Wikander TM, Ferdinandusse S, Duran M, Wanders RJ, Faust PL. Bile acid treatment alters hepatic disease and bile acid transport in peroxisome‐deficient PEX2 Zellweger mice. Hepatology. 2007 Apr;45(4):982-97.