Satellite Cell
Original Editor - Lucinda hampton
Top Contributors - Lucinda hampton and Kim Jackson
Introduction[edit | edit source]
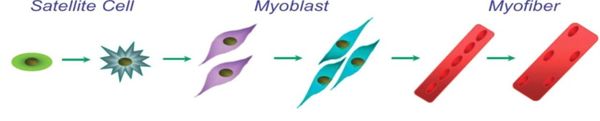
Skeletal muscle satellite cells are considered to play a crucial role in muscle fiber maintenance, repair and remodeling[1].
Satellite cells
- Mononucleated cells “wedged” between the basement membrane and plasma membrane of the muscle fiber.
- Act as stem cells and are responsible for the further growth and development of skeletal muscles.
- In response to injury are able to activate and re-enter the cell cycle, creating new cells to repair and create nascent muscle fibres while preserving a small population that can return to quiescence for future regenerative demands.
- Responsible for muscle growth as well as for the repair of muscle in response to stresses such as exercise, injury, or disease[2].
- The precise role of satellite cells in the development of muscle fiber atrophy with age remains unresolved[1]
Physiology[edit | edit source]
Homeostatic quiescence and activation is critical for establishing short- and long-term regenerative potential of the muscle by activating satellite cells and creating new progenitors for muscle repair, while preserving a small population of stem and committed satellite cells that can enter quiescence for the future regenerative demands.
- Under homeostatic conditions, the satellite cell niche provides cues to actively maintain quiescence signalling pathways while suppressing activation pathways.
- Upon injury, these cues activate satellite cells to prepare for the anticipated demands of regeneration. Inflammatory cytokines are also released to provide chemotaxic cues for leucocytes (eg macrophages) required to clear debris and remove toxic waste.
- Preservation of the satellite cell pool is critical for maintaining regeneration potential and also provides cues for satellite cells to return to quiescence when regeneration is complete.
- One of the most effective stimuli to induce satellite cell activation in adult muscles is exercise. Resistance or workload exercise is known to activate and increase numbers of satellite cells, as well as promote myonuclear expansion and hypertrophy
- Endurance exercise also promotes changes in satellite cell function, stemness, self‐renewal, and differentiation.[3]
Exercises Role[edit | edit source]
Satellite cells go into a state of dormancy if the body deems them unnecessary due to a sedentary lifestyle.
- Researchers have found that satellite cells are activated through various signaling pathways as a response to exercise.
- If they remain in a dormant state for too long, they can become damaged as they fill with cellular trash.
Satellite cells increase their cytoplasm during rounds of proliferation, which dilutes cellular waste and dysfunctional organelles between daughter cells. It is evident that waste and DNA damage accrued within periods of satellite cell quiescence are most effectively managed during periods of activation.
- Frequent activation of muscle satellite cells through exercise is a key process required to offset age-related waste/damage accumulation that leads to senescence.
- Physical inactivity becomes more detrimental with age as satellite cells accumulate higher levels of cellular waste and DNA damage during longer periods of deep quiescence.
- Keeping dormant satellite cells healthy requires regular activation.
The satellite cell pool can increase as early as 4 days following a single bout of exercise and is maintained at higher level following several weeks of training. Cessation of training is associated with a gradual reduction of the previously enhanced satellite cell pool.[4]
Ageing and Satellite Cells[edit | edit source]
In aged muscle, satellite cells that experience prolonged quiescence will undergo programmed cellular senescence, an irreversible non-dividing state that handicaps the regenerative capabilities of muscle. In the older person, training counteracts the normal decline in satellite cell number seen with ageing.
- As satellite cells age and their regenerative potential declines, they convert from a myogenic to a fibrogenic lineage.[5]
- Currently, research efforts are underway to elucidate whether exercise training actually confers a survival benefit to the satellite cells and whether exercise can restore satellite cell function either directly via modification of the proteasome or through epigenetic modifications. [1]
People need to activate their muscles on a regular basis once they reach age 30, or face the possibility of losing the ability to regenerate muscle mass as they age. Even with regular exercise, people will experience age-related skeletal muscle defects (sarcopenia) and will experience some loss in satellite cell viability—making regular exercise all the more important for people as they grow older.[6]
Physiotherapy[edit | edit source]
The need to promote exercise though out the life span is highlighted by these findings.
- Education plays a key role
- It has been demonstrated that exercise training can mitigate at least some of the negative effects of aging on satellite cell number and particularly skeletal muscle as a whole[1].
See: Physical Activity; Physical Activity in Ageing and Falls; Age and Exercise; Sarcopenia
Further Research[edit | edit source]
- Over the past decade, considerable advances in our understanding of skeletal muscle satellite cells during muscle fiber repair and remodeling in human skeletal muscle have been made. Evidence from in vivo human studies suggests that satellite cells play a key role, not only in growth and hypertrophy but also in adaptation and remodeling in response to damaging and non-damaging exercise[1].
- Many additional mechanisms behind satellite cell function decline in ageing remain to be discovered. Currently, our understanding of satellite cell behaviour in response to exercise is relatively limited compared with more severe models of injury and future research is needed.[6]
References[edit | edit source]
- ↑ 1.0 1.1 1.2 1.3 1.4 Snijders T, Nederveen JP, McKay BR, Joanisse S, Verdijk LB, van Loon LJ, Parise G. Satellite cells in human skeletal muscle plasticity. Frontiers in physiology. 2015 Oct 21;6:283.Available from: https://www.frontiersin.org/articles/10.3389/fphys.2015.00283/full#h8(accessed 21.3.2021)
- ↑ Chang NC, Rudnicki MA. Satellite cells: the architects of skeletal muscle. Current topics in developmental biology. 2014 Jan 1;107:161-81.Available from:https://www.sciencedirect.com/topics/medicine-and-dentistry/satellite-cell (accessed 21.3.2021)
- ↑ Abreu P, Kowaltowski AJ. Satellite cell self‐renewal in endurance exercise is mediated by inhibition of mitochondrial oxygen consumption. Journal of cachexia, sarcopenia and muscle. 2020 Dec;11(6):1661-76.Available from:https://onlinelibrary.wiley.com/doi/full/10.1002/jcsm.12601 (accessed 21.3.2021)
- ↑ Kadi F, Charifi N, Denis C, Lexell J, Andersen JL, Schjerling P, Olsen S, Kjaer M. The behaviour of satellite cells in response to exercise: what have we learned from human studies?. Pflügers Archiv. 2005 Nov;451(2):319-27.Available from: https://www.researchgate.net/publication/7668338_The_behaviour_of_satellite_cells_in_response_to_exercise_What_have_we_learned_from_human_studies(accessed 21.3.2021)
- ↑ Sher RB, Cox GA, Ackert-Bicknell C. Development and disease of mouse muscular and skeletal systems.Available from:https://www.sciencedirect.com/topics/medicine-and-dentistry/satellite-cell (accessed 21.3.2021)
- ↑ 6.0 6.1 Chen W, Datzkiw D, Rudnicki MA. Satellite cells in ageing: Use it or lose it. Open biology. 2020 May 20;10(5):200048.Available from: https://royalsocietypublishing.org/doi/10.1098/rsob.200048(accessed 21.3.2021)