Basal Ganglia: Difference between revisions
No edit summary |
No edit summary |
||
| (7 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
<div class="editorbox"> '''Original Editor '''- [[User:Lucinda hampton|Lucinda Hampton]] '''Top Contributors''' - {{Special:Contributors/{{FULLPAGENAME}}}}</div> | <div class="editorbox"> '''Original Editor '''- [[User:Lucinda hampton|Lucinda Hampton]] '''Top Contributors''' - {{Special:Contributors/{{FULLPAGENAME}}}}</div> | ||
<div class="noeditbox">This article or area is currently under construction and may only be partially complete. Please come back soon to see the finished work! ({{REVISIONDAY}}/{{REVISIONMONTH}}/{{REVISIONYEAR}})</div> | |||
== Introduction to the Basal Ganglia == | == Introduction to the Basal Ganglia == | ||
[[File:Overview of the basal ganglia - Kenhub.png|alt=Overview of the basal ganglia (corpus striatum) - lateral view|300x300px|Overview of the basal ganglia (corpus striatum) - lateral view|thumb]] | |||
The '''basal ganglia''' are a group of subcortical nuclei, located at the base of the forebrain,<ref>Yanagisawa N. [https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6117491/ Functions and dysfunctions of the basal ganglia in humans.] Proc Jpn Acad Ser B Phys Biol Sci. 2018;94(7):275-304. </ref> that is primarily involved in motor control, as well as motor learning, executive functions and emotional behaviours. It also plays an important role in reward and reinforcement, addictive behaviours and habit formation.<ref name=":0">Lanciego JL, Luquin N, Obeso JA. [https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3543080/ Functional neuroanatomy of the basal ganglia.] Cold Spring Harbor perspectives in medicine. 2012 Dec 1;2(12):a009621.</ref> | |||
Basal ganglia network dysfunction leads to various movement disorders,<ref name=":0" /> such as [[Parkinson's]] and [[Huntington Disease|Huntington's Disease.]] | |||
== Structure == | == Structure of the Basal Ganglia == | ||
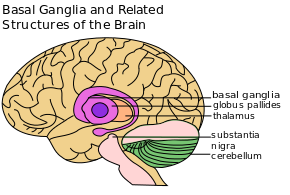
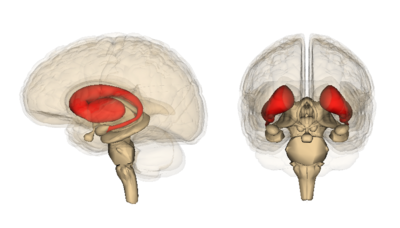
[[File:PD Basal Ganglia etc.png|alt=A line diagram of the main structures of the basal ganglia|left|frameless]][[File:PD Basal ganglia .jpg|middle|frameless]] | [[File:PD Basal Ganglia etc.png|alt=A line diagram of the main structures of the basal ganglia|left|frameless|Basal ganglia.]][[File:PD Basal ganglia .jpg|middle|frameless]] | ||
The '''corpus striatum''' is the largest component of the basal ganglia'''.''' It contains the '''caudate nucleus''' and '''lenticular nuclei''', which is made up of the '''putamen''', '''globus pallidus externus''', and '''globus pallidus internus.''' The '''subthalamic nucleus''' (STN), and the '''substantia nigra''' (SN) are also components of the basal ganglia. These structures work together to promote or inhibit movement.<ref name=":1">Young CB, Sonne J. [https://www.ncbi.nlm.nih.gov/books/NBK537141/ Neuroanatomy, basal ganglia.] InStatPearls [Internet] 2018 Dec 28. StatPearls Publishing.</ref>[[File:Striatum.png|400x400px|thumb|Doral Corpus Striatum ]] | |||
[[File:Striatum.png|400x400px|thumb|Doral Corpus Striatum ]] | * '''Corpus Striatum''': a heterogeneous structure with a volume of around 10 cm<sup>3</sup>. It receives afferent inputs from various cortical and subcortical structures and sends outputs to different basal ganglia nuclei.<ref name=":0" /> There are two main divisions in the corpus striatum: | ||
** '''Dorsal Striatum''' (DS) (shown in red in the image): mostly involved in the control of conscious motor movements and executive functions. The dorsal striatum consists of the caudate nucleus and the putamen. The internal capsule is a white matter nerve tract in the dorsal striatum that separates the caudate nucleus from the putamen. | |||
** '''Ventral Striatum''': involved in the limbic functions of reward and aversion. It is made up of the nucleus accumbens and the olfactory tubercle.<ref name=":1" /> | |||
* Internal and external segments of the '''Globus Pallidus''': together, the globus pallidus and putamen form the lentiform (or lenticular) nucleus.<ref>Javed N, Cascella M. [https://www.ncbi.nlm.nih.gov/books/NBK557755/ Neuroanatomy, Globus Pallidus.] [Updated 2023 Feb 20]. In: StatPearls [Internet].</ref> | |||
* '''Subthalamic Nucleus''' (STN): a lens-shaped cell group that makes up a large part of the subthalamus.<ref>Basinger H, Joseph J. Neuroanatomy, Subthalamic Nucleus. [Updated 2022 Oct 31]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK559002/</ref> | |||
* '''Substantia Nigra''' (SN) - (which means "black substance" in Latin): while the substantia nigra is located in the midbrain, it is considered part of the basal ganglia. This long nucleus has two parts: 1) pars compacta and 2) pars reticulata. Degeneration of the pars compacta decreases the amount of available dopamine.<ref>Jacobs LK, Sapers BL. [https://www.sciencedirect.com/science/article/pii/B9780443068980000335 Neurological Disease.] InPerioperative Medicine 2011 (pp. 343-359). Springer, London.</ref><br /> | |||
The two optional videos below provide further information on the structure and function of the basal ganglia:<div class="row"> | |||
The | |||
<div class="col-md-6"> {{#ev:youtube|OD2KPSGZ1No|250}} <div class="text-right"><ref>Neuroscientifically Challenged Basal ganglia . Available from: https://www.youtube.com/watch?v=OD2KPSGZ1No [last accessed 14/01/2020]</ref></div></div> | <div class="col-md-6"> {{#ev:youtube|OD2KPSGZ1No|250}} <div class="text-right"><ref>Neuroscientifically Challenged Basal ganglia . Available from: https://www.youtube.com/watch?v=OD2KPSGZ1No [last accessed 14/01/2020]</ref></div></div> | ||
<div class="col-md-6"> {{#ev:youtube|mNc-6q6YAAw|250}} <div class="text-right"><ref>Armando Hasudungan. The Basal Ganglia Clinical Anatomy. Available from: https://www.youtube.com/watch?v=mNc-6q6YAAw [last accessed 05/01/2024]</ref></div></div> | <div class="col-md-6"> {{#ev:youtube|mNc-6q6YAAw|250}} <div class="text-right"><ref>Armando Hasudungan. The Basal Ganglia Clinical Anatomy. Available from: https://www.youtube.com/watch?v=mNc-6q6YAAw [last accessed 05/01/2024]</ref></div></div> | ||
| Line 25: | Line 25: | ||
== Basal Ganglia | == Function of the Basal Ganglia == | ||
The classical basal ganglia model suggested that "information flows through the basal ganglia back to the cortex through two pathways with opposing effects for the proper execution of movement."<ref name=":02">Lanciego JL, Luquin N, Obeso JA. [https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3543080/ Functional neuroanatomy of the basal ganglia.] Cold Spring Harbor perspectives in medicine. 2012 Dec 1;2(12):a009621.</ref> In this "loop", cortical input is sent to the basal ganglia, where it is modified and then sent back to the cortex. As a result of this feedback, motor activity is either facilitated or inhibited.<ref name=":02" /> | |||
This model has been reviewed and updated. It is now believed that the basal ganglia have "multiple parallel loops and re-entering circuits whereby motor, associative, and limbic territories are engaged mainly in the control of movement, behaviour, and emotions."<ref name=":02" /> | |||
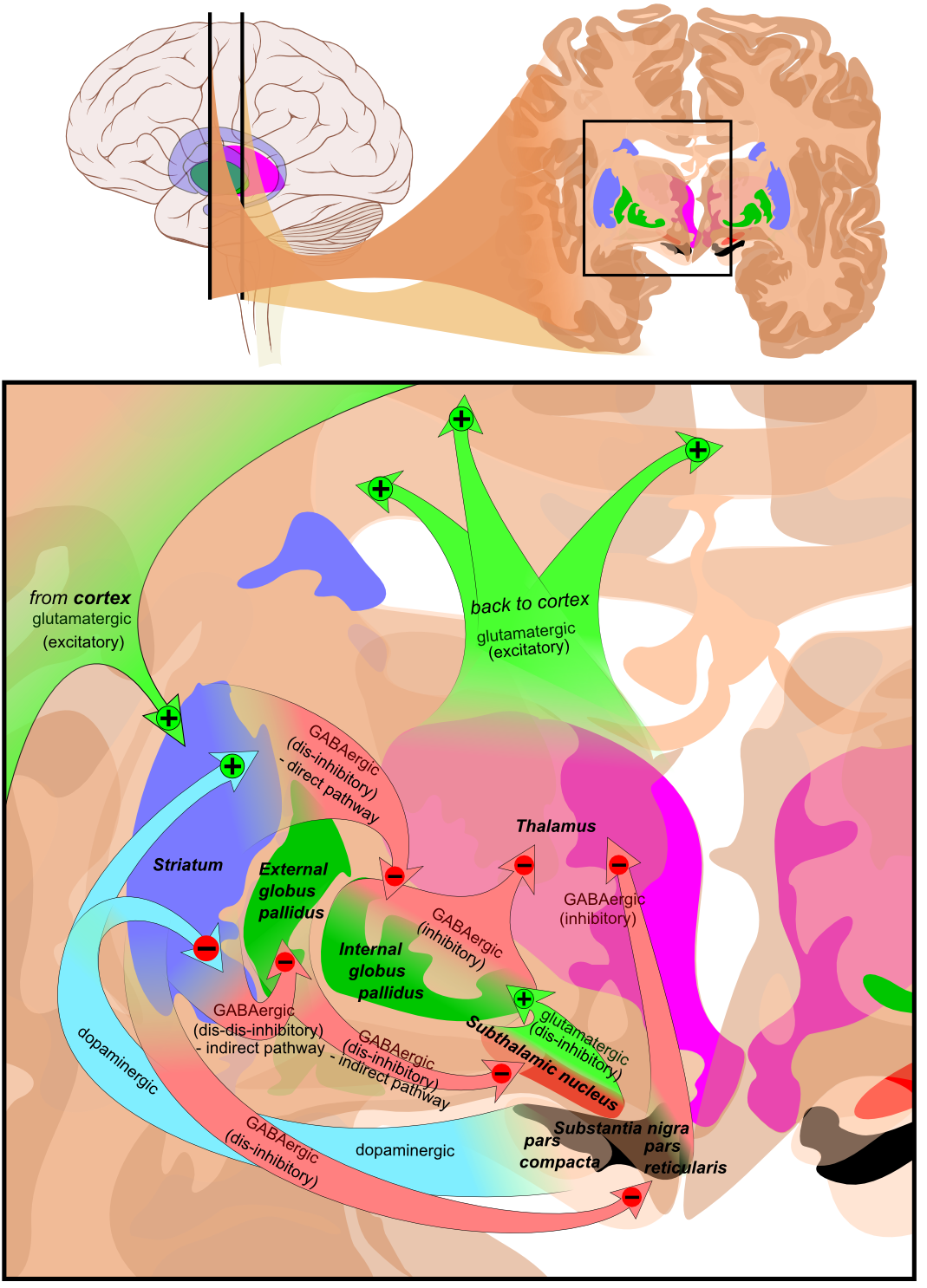
The following image illustrates various excitatory and inhibitory pathways from the basal ganglia to the cortex.<ref name=":0" /> | |||
[[File:Basal ganglia circuits.png|center|alt=This is a diagram of the flow of excitatory and inhibitory pathways through the basal ganglia.|frame|Basal ganglia circuits]] | [[File:Basal ganglia circuits.png|center|alt=This is a diagram of the flow of excitatory and inhibitory pathways through the basal ganglia.|frame|Basal ganglia circuits]] | ||
== | == Associated Conditions == | ||
Dysfunction of the basal ganglia is associated with specific movement disorders, and can cause issues such as (1) tremor, (2) involuntary muscle movements, (3) abnormal increase in tone, (4) difficulty initiating movements, and (5) abnormal posture. The following sections discuss movement disorders associated with basal ganglia dysfunction.<ref name=":1" /> | |||
=== Parkinson's === | |||
[[Parkinson's]] is the second most common neurodegenerative disorder. Its aetiology is multifactorial, with both genetic and environmental risk factors identified.<ref>Ben-Shlomo Y, Darweesh S, Llibre-Guerra J, Marras C, San Luciano M, Tanner C. The epidemiology of Parkinson's disease. The Lancet. 2024;403(10423):283-92.</ref> Parkinson's is characterised "by the progressive loss of dopaminergic neurons and the formation of Lewy bodies in the affected brain areas".<ref name=":3">Zhou, ZD, Yi LX, Wang DQ, Lim TM, Tan EK. [https://translationalneurodegeneration.biomedcentral.com/articles/10.1186/s40035-023-00378-6 Role of dopamine in the pathophysiology of Parkinson’s disease]. Transl Neurodegener. 2023;12(44). </ref> Because of the degeneration of dopaminergic neurons, there is less dopamine available in the substantia nigra and striatum, which causes various clinical signs of Parkinson's, such as:<ref name=":3" /> | |||
* tremor | |||
[[ | * postural instability | ||
* [[bradykinesia]] | |||
* muscle rigidity | |||
''' | === Huntington's Disease === | ||
[[Huntington Disease|Huntington's disease]]<ref name=":4">Matz OC, Spocter M. The Effect of Huntington's Disease on the Basal Nuclei: A Review. Cureus. 2022;14(4):e24473.</ref> is a progressive, inherited neurodegenerative disease. It is associated with neuropsychiatric symptoms, movement disorders (usually chorea) and cognitive impairment / dementia.<ref name=":5">Stoker TB, Mason SL, Greenland JC, Holden ST, Santini H, Barker RA. [https://pn.bmj.com/content/practneurol/22/1/32.full.pdf Huntington's disease: diagnosis and management]. Pract Neurol. 2022 Feb;22(1):32-41. </ref><ref>Medina A, Mahjoub Y, Shaver L, Pringsheim T. [https://movementdisorders.onlinelibrary.wiley.com/doi/full/10.1002/mds.29228 Prevalence and incidence of Huntington's disease: an updated systematic review and meta-analysis]. Mov Disord. 2022 Dec;37(12):2327-35. </ref> Huntington's disease occurs in individuals with a CAG expansion in the huntingtin gene.<ref name=":5" /> There is neurodegeneration in many parts of the brain, the striatum<ref name=":5" /> and cortex are particularly affected.<ref name=":6">Kim A, Lalonde K, Truesdell A, Gomes Welter P, Brocardo PS, Rosenstock TR, Gil-Mohapel J. [https://www.mdpi.com/1422-0067/22/16/8363 New avenues for the treatment of Huntington's disease]. Int J Mol Sci. 2021 Aug 4;22(16):8363. </ref> Symptoms usually start in individuals aged between 30-50 years. A key motor symptom is chorea (i.e. "brief, involuntary movements that generally affect the trunk, face, and arms"<ref name=":6" />). Chorea affects voluntary movements, ultimately affecting walking, speaking and swallowing.<ref name=":6" /> | |||
Other motor signs include:<ref name=":6" /> | |||
* bradykinesia | |||
* dystonia | |||
* hyperreflexia | |||
* decreased speed of eye saccades | |||
Cognitive impairments include:<ref name=":6" /> | |||
* decreased executive function (difficulty with attention, concentration, multi-tasking, decision making) | |||
* depression | |||
* loss of memory | |||
* decreased insight | |||
The most common psychiatric conditions are:<ref name=":6" /> | |||
* | * depression | ||
* | * irritability | ||
* | * increased impulsivity | ||
=== Hemiballism === | === Hemiballism === | ||
Hemiballism<ref name=": | Hemiballism (from the verb “to throw” in Greek) is a rare movement disorder that causes "high amplitude movement of an entire limb or limbs on one side of the body."<ref name=":7">Hawley JS, Weiner WJ. Hemiballismus: current concepts and review. Parkinsonism Relat Disord. 2012 Feb;18(2):125-9.</ref> Hemiballism can be caused by several conditions, but acute cases are often associated with focal lesions in the contralateral basal ganglia and subthalamic nucleus lesion.<ref name=":7" /> Individuals with hemiballism tend to have a good prognosis.<ref name=":7" /> | ||
=== Tourette Syndrome === | === Tourette Syndrome === | ||
Tourette syndrome | Tourette syndrome is a persistent neurodevelopmental condition associated with motor and phonic tics.<ref name=":2">Johnson KA, Worbe Y, Foote KD, Butson CR, Gunduz A, Okun MS. Tourette syndrome: clinical features, pathophysiology, and treatment. Lancet Neurol. 2023 Feb;22(2):147-58. </ref> While its pathophysiology is not fully understood, tics may be due to "inhibitory dysfunction within the sensorimotor cortico-basal ganglia network".<ref name=":2" /> Tourette syndrome can significantly impact quality of life, and treatment options are expanding.<ref name=":2" /> | ||
== References == | == References == | ||
Latest revision as of 03:36, 26 June 2024
Introduction to the Basal Ganglia[edit | edit source]
The basal ganglia are a group of subcortical nuclei, located at the base of the forebrain,[1] that is primarily involved in motor control, as well as motor learning, executive functions and emotional behaviours. It also plays an important role in reward and reinforcement, addictive behaviours and habit formation.[2]
Basal ganglia network dysfunction leads to various movement disorders,[2] such as Parkinson's and Huntington's Disease.
Structure of the Basal Ganglia[edit | edit source]
 The corpus striatum is the largest component of the basal ganglia. It contains the caudate nucleus and lenticular nuclei, which is made up of the putamen, globus pallidus externus, and globus pallidus internus. The subthalamic nucleus (STN), and the substantia nigra (SN) are also components of the basal ganglia. These structures work together to promote or inhibit movement.[3]
The corpus striatum is the largest component of the basal ganglia. It contains the caudate nucleus and lenticular nuclei, which is made up of the putamen, globus pallidus externus, and globus pallidus internus. The subthalamic nucleus (STN), and the substantia nigra (SN) are also components of the basal ganglia. These structures work together to promote or inhibit movement.[3]
- Corpus Striatum: a heterogeneous structure with a volume of around 10 cm3. It receives afferent inputs from various cortical and subcortical structures and sends outputs to different basal ganglia nuclei.[2] There are two main divisions in the corpus striatum:
- Dorsal Striatum (DS) (shown in red in the image): mostly involved in the control of conscious motor movements and executive functions. The dorsal striatum consists of the caudate nucleus and the putamen. The internal capsule is a white matter nerve tract in the dorsal striatum that separates the caudate nucleus from the putamen.
- Ventral Striatum: involved in the limbic functions of reward and aversion. It is made up of the nucleus accumbens and the olfactory tubercle.[3]
- Internal and external segments of the Globus Pallidus: together, the globus pallidus and putamen form the lentiform (or lenticular) nucleus.[4]
- Subthalamic Nucleus (STN): a lens-shaped cell group that makes up a large part of the subthalamus.[5]
- Substantia Nigra (SN) - (which means "black substance" in Latin): while the substantia nigra is located in the midbrain, it is considered part of the basal ganglia. This long nucleus has two parts: 1) pars compacta and 2) pars reticulata. Degeneration of the pars compacta decreases the amount of available dopamine.[6]
The two optional videos below provide further information on the structure and function of the basal ganglia:
Function of the Basal Ganglia[edit | edit source]
The classical basal ganglia model suggested that "information flows through the basal ganglia back to the cortex through two pathways with opposing effects for the proper execution of movement."[9] In this "loop", cortical input is sent to the basal ganglia, where it is modified and then sent back to the cortex. As a result of this feedback, motor activity is either facilitated or inhibited.[9]
This model has been reviewed and updated. It is now believed that the basal ganglia have "multiple parallel loops and re-entering circuits whereby motor, associative, and limbic territories are engaged mainly in the control of movement, behaviour, and emotions."[9]
The following image illustrates various excitatory and inhibitory pathways from the basal ganglia to the cortex.[2]
Associated Conditions[edit | edit source]
Dysfunction of the basal ganglia is associated with specific movement disorders, and can cause issues such as (1) tremor, (2) involuntary muscle movements, (3) abnormal increase in tone, (4) difficulty initiating movements, and (5) abnormal posture. The following sections discuss movement disorders associated with basal ganglia dysfunction.[3]
Parkinson's[edit | edit source]
Parkinson's is the second most common neurodegenerative disorder. Its aetiology is multifactorial, with both genetic and environmental risk factors identified.[10] Parkinson's is characterised "by the progressive loss of dopaminergic neurons and the formation of Lewy bodies in the affected brain areas".[11] Because of the degeneration of dopaminergic neurons, there is less dopamine available in the substantia nigra and striatum, which causes various clinical signs of Parkinson's, such as:[11]
- tremor
- postural instability
- bradykinesia
- muscle rigidity
Huntington's Disease[edit | edit source]
Huntington's disease[12] is a progressive, inherited neurodegenerative disease. It is associated with neuropsychiatric symptoms, movement disorders (usually chorea) and cognitive impairment / dementia.[13][14] Huntington's disease occurs in individuals with a CAG expansion in the huntingtin gene.[13] There is neurodegeneration in many parts of the brain, the striatum[13] and cortex are particularly affected.[15] Symptoms usually start in individuals aged between 30-50 years. A key motor symptom is chorea (i.e. "brief, involuntary movements that generally affect the trunk, face, and arms"[15]). Chorea affects voluntary movements, ultimately affecting walking, speaking and swallowing.[15]
Other motor signs include:[15]
- bradykinesia
- dystonia
- hyperreflexia
- decreased speed of eye saccades
Cognitive impairments include:[15]
- decreased executive function (difficulty with attention, concentration, multi-tasking, decision making)
- depression
- loss of memory
- decreased insight
The most common psychiatric conditions are:[15]
- depression
- irritability
- increased impulsivity
Hemiballism[edit | edit source]
Hemiballism (from the verb “to throw” in Greek) is a rare movement disorder that causes "high amplitude movement of an entire limb or limbs on one side of the body."[16] Hemiballism can be caused by several conditions, but acute cases are often associated with focal lesions in the contralateral basal ganglia and subthalamic nucleus lesion.[16] Individuals with hemiballism tend to have a good prognosis.[16]
Tourette Syndrome[edit | edit source]
Tourette syndrome is a persistent neurodevelopmental condition associated with motor and phonic tics.[17] While its pathophysiology is not fully understood, tics may be due to "inhibitory dysfunction within the sensorimotor cortico-basal ganglia network".[17] Tourette syndrome can significantly impact quality of life, and treatment options are expanding.[17]
References[edit | edit source]
- ↑ Yanagisawa N. Functions and dysfunctions of the basal ganglia in humans. Proc Jpn Acad Ser B Phys Biol Sci. 2018;94(7):275-304.
- ↑ 2.0 2.1 2.2 2.3 Lanciego JL, Luquin N, Obeso JA. Functional neuroanatomy of the basal ganglia. Cold Spring Harbor perspectives in medicine. 2012 Dec 1;2(12):a009621.
- ↑ 3.0 3.1 3.2 Young CB, Sonne J. Neuroanatomy, basal ganglia. InStatPearls [Internet] 2018 Dec 28. StatPearls Publishing.
- ↑ Javed N, Cascella M. Neuroanatomy, Globus Pallidus. [Updated 2023 Feb 20]. In: StatPearls [Internet].
- ↑ Basinger H, Joseph J. Neuroanatomy, Subthalamic Nucleus. [Updated 2022 Oct 31]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK559002/
- ↑ Jacobs LK, Sapers BL. Neurological Disease. InPerioperative Medicine 2011 (pp. 343-359). Springer, London.
- ↑ Neuroscientifically Challenged Basal ganglia . Available from: https://www.youtube.com/watch?v=OD2KPSGZ1No [last accessed 14/01/2020]
- ↑ Armando Hasudungan. The Basal Ganglia Clinical Anatomy. Available from: https://www.youtube.com/watch?v=mNc-6q6YAAw [last accessed 05/01/2024]
- ↑ 9.0 9.1 9.2 Lanciego JL, Luquin N, Obeso JA. Functional neuroanatomy of the basal ganglia. Cold Spring Harbor perspectives in medicine. 2012 Dec 1;2(12):a009621.
- ↑ Ben-Shlomo Y, Darweesh S, Llibre-Guerra J, Marras C, San Luciano M, Tanner C. The epidemiology of Parkinson's disease. The Lancet. 2024;403(10423):283-92.
- ↑ 11.0 11.1 Zhou, ZD, Yi LX, Wang DQ, Lim TM, Tan EK. Role of dopamine in the pathophysiology of Parkinson’s disease. Transl Neurodegener. 2023;12(44).
- ↑ Matz OC, Spocter M. The Effect of Huntington's Disease on the Basal Nuclei: A Review. Cureus. 2022;14(4):e24473.
- ↑ 13.0 13.1 13.2 Stoker TB, Mason SL, Greenland JC, Holden ST, Santini H, Barker RA. Huntington's disease: diagnosis and management. Pract Neurol. 2022 Feb;22(1):32-41.
- ↑ Medina A, Mahjoub Y, Shaver L, Pringsheim T. Prevalence and incidence of Huntington's disease: an updated systematic review and meta-analysis. Mov Disord. 2022 Dec;37(12):2327-35.
- ↑ 15.0 15.1 15.2 15.3 15.4 15.5 Kim A, Lalonde K, Truesdell A, Gomes Welter P, Brocardo PS, Rosenstock TR, Gil-Mohapel J. New avenues for the treatment of Huntington's disease. Int J Mol Sci. 2021 Aug 4;22(16):8363.
- ↑ 16.0 16.1 16.2 Hawley JS, Weiner WJ. Hemiballismus: current concepts and review. Parkinsonism Relat Disord. 2012 Feb;18(2):125-9.
- ↑ 17.0 17.1 17.2 Johnson KA, Worbe Y, Foote KD, Butson CR, Gunduz A, Okun MS. Tourette syndrome: clinical features, pathophysiology, and treatment. Lancet Neurol. 2023 Feb;22(2):147-58.