Arterial Blood Gases: Difference between revisions
No edit summary |
(content) |
||
| Line 95: | Line 95: | ||
== Respiratory Acidosis == | == Respiratory Acidosis == | ||
caused by inadequate ventilation leading to the retention of carbon dioxide and an increase in free hydrogen ions. | Respiratory acidosis is caused by inadequate alveolar ventilation leading to the retention of carbon dioxide and an increase in free hydrogen ions. | ||
* Decreased pH | * Decreased pH | ||
* Increased PaCO2 | * Increased PaCO2 | ||
| Line 119: | Line 119: | ||
== Metabolic Acidosis == | == Metabolic Acidosis == | ||
involves excess fixed acid production, i.e. lactate or loss of HCO<sub>3</sub>. | Metabolic acidosis involves excess fixed acid production, i.e. lactate or loss of HCO<sub>3</sub>. | ||
* Decreased pH | * Decreased pH | ||
* Decreased HCO3 | * Decreased HCO3 | ||
| Line 141: | Line 141: | ||
== Respiratory Alkalosis == | == Respiratory Alkalosis == | ||
caused by over excretion of carbon dioxide leading to a reduction in free hydrogen ions and an alkalotic state. | Respiratory alkalosis is caused by over excretion of carbon dioxide (hyperventilation) resulting in more CO2 than normal being exhaled. Thus, leading to a reduction in free hydrogen ions and an alkalotic state. | ||
* Increased pH | * Increased pH | ||
* Decreased PaCO2 | * Decreased PaCO2 | ||
| Line 161: | Line 161: | ||
== Metabolic Alkalosis == | == Metabolic Alkalosis == | ||
Metabolic alkalosis occurs as a result of decreased hydrogen ion concentration which leads to increased bicarbonate, or alternatively a direct result of increased bicarbonate concentrations. | |||
* Increased pH | * Increased pH | ||
* Increased HCO3 | * Increased HCO3 | ||
Revision as of 21:29, 8 March 2018
Original Editor - Scott Buxton
Top Contributors - Adam Vallely Farrell, Kim Jackson, Uchechukwu Chukwuemeka, Scott Buxton, Lucinda hampton, Rachael Lowe, Admin, Joao Costa, 127.0.0.1, Laura Ritchie, Naomi O'Reilly, Abbey Wright and Angeliki Chorti
Arterial Blood Gases[edit | edit source]
Arterial blood gases (ABG's) is a blood test which is used to give an indication of ventilation, gas exchange and acid-base status and is taken from an arterial blood supply[1]. It should be noted that it is not to be confused with venous blood gases which are used when arterial supply is not available or unreliable due to disease. The line in is usually interted in the radial artery located at the wrist but is also sometimes used in the femoral artery in the groin[1]. This is important to consider when moving and handling a patient as it is easy to catch a line on an obect or clothing and care needs to be taken.
It can be argued that one of the most important clinical uses of ABG analysis is to assess if a patient is in Type 1 or Type 2 respiratory failure and it is important that you become able to quickly and correctly interpret this.
ABGs give us information about the activity in both the respiratory system and the 'metabolic' system. If one system is disturbed, the other will try to restore the balance. Both systems work together in an attempt to keep pH in the normal range. They are commonly used to; identify acid/base disorders, identify gas exchange problems, monitor the effects of oxygen therapy.
ABG Definitions[edit | edit source]
- PH: The measure of hydrogen ions in the blood
- PaC02: Partial pressure of Carbon Dioxide in the blood, the acidic element of the balance
- Acidic component
- Indicator of respiratory function
- Changes rapidly to compensate
- Pa02: Partial pressure of oxygen in the blood.
- HCO3-: Bicarbonate ion concentration in the blood, the basic element of the balance
- Basic/Alkaline component
- Indicator of metabolic function
- Compensation is slower
- BE: Base Excess = quantity of strong acid or base that is required to restore pH to normal
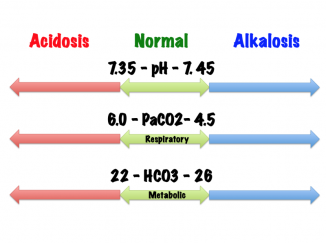
Normative Values [2][edit | edit source]
| Arterial Blood Analysis | Reference Ranges | Venous Blood analysis | Reference Ranges |
|---|---|---|---|
| pH | 7.35 - 7.45 | pH | 7.31 - 7.41 |
| PaO2 |
10.7 - 13.3 kPa |
PO2 |
5.0 - 5.6 kPa |
| PaCO2 | 4.7 - 6.0 kPa | PCO2 | 5.6 - 6.7 kPa |
|
Bicarbonate (HCO3-) |
22 - 26 mmol/L | ||
| Base Excess | -2 to +2 |
Acid-Base Balance[edit | edit source]
- pH
- Reflects acid-base balance and responds to metabolic and respiratory change. Body cells and chemical reactions are acutely sensitive to the pH of their environment.
- Lower pH = Acidic
- Higher pH= Alkaline
- Reflects acid-base balance and responds to metabolic and respiratory change. Body cells and chemical reactions are acutely sensitive to the pH of their environment.
- Regulation
- Acid-base balance is disturbed if; CO2 removal by the lungs is abnormal, production of acid from tissues is abnormal, and removal of acid is abnormal.
- Deviation from normal pH is resisted by 3 mechanisms;
- The Buffering system
- Acts like a chemical sponge and neutralises acids and bases.
- The Lungs
- The respiratory component that reacts if the buffering system is not enough and the lungs help my regulating CO2.
- The Kidneys
- The Metabolic component and is the last mechanism to work and begins to eliminate acid.
Respiratory Acidosis[edit | edit source]
Respiratory acidosis is caused by inadequate alveolar ventilation leading to the retention of carbon dioxide and an increase in free hydrogen ions.
- Decreased pH
- Increased PaCO2
- Causes
- Hypoventilation
- Acute Lunge Injury
- Upper airway obstruction
- Lower airway obstruction
- Impaired alveolar filling
- Chronic Lung Disease
- Neuromuscular disorders
- Obesity
- CNS Depression
- Symptoms
- Headache
- Anxiety
- Blurred vision
- Restlessness
- Drowsiness
- Tremors
- Delirium
- Coma
Metabolic Acidosis[edit | edit source]
Metabolic acidosis involves excess fixed acid production, i.e. lactate or loss of HCO3.
- Decreased pH
- Decreased HCO3
- Causes
- Body produces too much acid or the kidneys are not removing it;
- Renal disease
- Liver disease
- Lactic acidosis
- Prolonged lack of oxygen
- Shock
- Posioning
- Medications
- Dehydration
- Diarrhoea
- Body produces too much acid or the kidneys are not removing it;
- Symptoms
- Rapid breathing
- Confusion
- Lethargy
- Cold, clammy skin
- Tachycardia and arrhythmia
Respiratory Alkalosis[edit | edit source]
Respiratory alkalosis is caused by over excretion of carbon dioxide (hyperventilation) resulting in more CO2 than normal being exhaled. Thus, leading to a reduction in free hydrogen ions and an alkalotic state.
- Increased pH
- Decreased PaCO2
- Causes
- Hyperventilation
- Acute asthma
- CNS disturbance
- High altitude
- Pneumonia
- Drugs
- Fever
- Sepsis
- Symptoms
- Diziness
- Peripheral paraesthesia
- Confusion
- Dry mouth
- Bloating
Metabolic Alkalosis[edit | edit source]
Metabolic alkalosis occurs as a result of decreased hydrogen ion concentration which leads to increased bicarbonate, or alternatively a direct result of increased bicarbonate concentrations.
- Increased pH
- Increased HCO3
- Causes
- Excess alkali administration
- Hyperkalaemia
- IV penicillin
- Re-feeding syndrome
- Massive blood transfusion
- Diuretic therapy
- Vomitting
- Symptoms
- Weakness
- Myalgia
- Polyuria
- Cardiac arrhythmias
- Hypoventilation
Respiratory Failure[edit | edit source]
Respiratory failure is not a disease but a consequence of the problems that interfere with the ability to breathe.
It is the inability to perform adequately the fundamental functions of respiration:
- To deliver oxygen to the blood
- To eliminate carbon dioxide from it
Interpretation of ABGs[edit | edit source]
- Look at the pH
- Increased = Alkalosis
- Decreased = Acidosis
- Look at the PaCO2
- Increased = Respiratory Acidosis
- Decreased = Respiratory Alkalosis
- Look at the HCO3
- Increased = Metabolic Alkalosis
- Decreased = Metabolic Acidosis
- Look at the O2
Compensation[edit | edit source]
The bodies pH is closely controlled and this is done through various mechanisms to maintain it at a constant value. It is important to note that the body will never overcompensate as the drivers for compensation cease as the pH returns to normal. In practice, compensation for an acidosis will not cause an alkalosis or visa versa.
If pH is NORMAL despite an abnormal PCO2 and HCO3 it must be compensated.
- Look at the pH - Which side of 7.4 is it?
- Look for the cause
- PCO2 goes in the opposite direction to pH
- HCO3 travels in the same direction as pH
- The other value is the compensator
Helpful guidelines[4]
1.A 1mmHg change in PaCO2 above or below 40 mmHg results in 0.008 unit change in pH in the opposite direction.
2.The PaCO2 will decrease by about 1 mmHg for every 1 mEq/L reduction in [HCO3-] below 24 mEq/L
3.A change in [HCO3-] of 10 mEq/L will result in a change in pH of approximately 0.15 pH units in the same direction.
The results should always be read and compared in reference to the patients previous ABG (if available) as you will then be able to assess a trend and make a more accurate assessment on whether you should treat or if your treatment has be successful or not.
Useful Resources[edit | edit source]
Associated Topics[edit | edit source]
- Oxygen Dissociation Cure
- Anemia
- Respiratory Failure
- Oxygen Therapy
References
[edit | edit source]
- ↑ 1.0 1.1 Hough A. Physiotherapy in Respiratory Care. An evidence based-approach to respiratory and cardiac managemenmt. 3rd ed. Cheltenham: Nelson Thomas Ltd. 2001
- ↑ Kenyon K, Kenyon J. The Physiotherapist's Pocketbook. Essential Facts at your Fingertips. 2nd ed. London: Churchill Livingstone, Elsevier. 2009.
- ↑ https://ed.ted.com/on/9q9pS35Z
- ↑ Stoeltin RK, Miller RD. Basics of Anesthesia, 5th ed. Philadelphia: Churchill Livingstone, Elsevier. 2007.