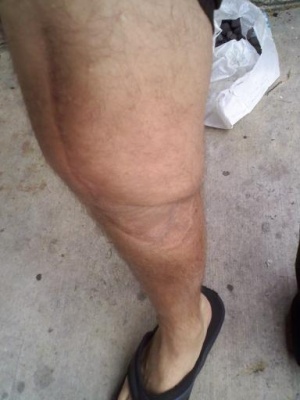
Osteosarcoma
Original Editors -Jody Swimmer from Bellarmine University's Pathophysiology of Complex Patient Problems project.
Lead Editors - Your name will be added here if you are a lead editor on this page. Read more.
Definition/Description[edit | edit source]
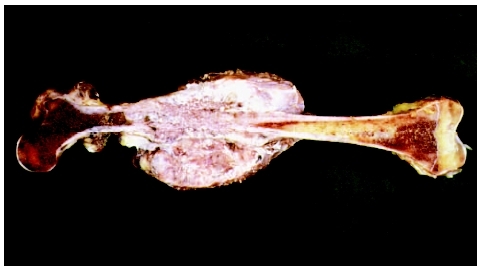
Osteosarcoma is also known as osteogenic sarcoma. Osteosarcoma is an extremely malignant primary cancer of long bones. Evidence of malignant osteoid bone and/or cartilage formation with destructive lesions and sclerosis is characteristic of the disease.[1] Differentiating osteosarcoma from other tumors is defined by the production of extensive, incompletely mineralized matrix that is seen with histological staining.[2]
Prevalence[edit | edit source]
Osteosarcoma accounts for 15% to 20% of all primary bone tumors and is the second most malignant condition of bone. It occurs most frequently in male adolescents and young adults under the age of 30, peaking in frequency during the adolescent growth spurt with another smaller peak in adults over the age of 50.[1] Review of data from the Surveillance, Epidemiology and End Results program of the NCI resulted in an estimate of 4.4 per million new cases of osteosarcoma each year in people aged newborn to 24 years.[3] “The U.S. Census Bureau estimates that there will be 110 million people in this age range in 2010, resulting in an incidence ofroughly 450 cases per year in children and young adults less than 25 years old. Osteosarcoma accounts for approximately 5% of childhood tumors. In children and adolescents, more than 50% of these tumors arise from the bones around the knee.”[3]
Characteristics/Clinical Presentation[edit | edit source]
The patient most often presents with pain prior to soft tissue swelling and an enlarging bone mass. This is due to the stretching of the periosteum which usually causes pain before the tumor is detected. Pain could also result from the weakening of the bone and the development of minute stress fractures. The majority of patients with osteosarcoma present with localized pain at the primary tumor site.[3]
The most commonly affected bones are the metaphyseal region of long bones such as the distal femur, proximal tibia, and proximal humerus although osteosarcoma can arise in any bone in the body.[4] Systemic symptoms such as weight loss, pallor and fever are uncommon. The patient may be a tall adolescent, with males presenting more often than females.
Distal locations of the tumor have a more favorable prognosis than proximal sites. “Axial skeleton primary tumors are associated with the greatest risk of progression and death, primarily related to the inability to achieve a complete surgical resection. Pelvic osteosarcomas make up 7%-9% of all osteosarcomas; survival rates for patients with pelvic primary tumors are 20% to 47%.”[3]
Associated Co-morbidities[edit | edit source]
Patients with Rothmund-Thomson Syndrome (RTS) have an increased risk of developing osteosarcoma when compared with the general population. They also develop osteosarcoma at an earlier age. RTS is also called poikiloderma congenital, which is a rare autosomal recessive condition.[3] This condition is characterized with skin issues such as atrophy, telangiectasias, pigmentation change, thinning or sparse hair, cataracts, small stature and skeletal anomalies.[1]
Other familial osteosarcoma syndromes which predispose an individual to osteosarcoma include: [5]
- Retinoblastoma
- Li-Fraumeni syndrome
- Werner syndrome
- Blooms syndrome
- Paget’s disease
- Fibrodysplasia
- Enchondromatosis (Ollier disease)
- Hereditary multiple exostoses.
“Another condition which may predispose the patient to the development of osteosarcoma is radiotherapy. For a sarcoma to be considered a post radiation tumor, the following conditions must be met:
- (1) A history of previous radiation
- (2) Development of the sarcoma in a bone that was in the field of radiation
- (3) A latent period between radiation and the development of osteosarcoma….most osteosarcomas arise between 5 and 10 years after radiation
- (4) Histologic difference from the previous tumor, which may have been irradiated. Most sarcomas arising in the field of radiation are high-grad osteosarcomas.” [6]
Among 220 children who were given Ra IV as therapy for tuberculosis, many developed osteosarcoma.[7]
Medications[edit | edit source]
- Chemotherapy – Combination therapy with gemcitabine and docetaxel in refractory bone sarcomas
- Immunotherapy - Interferon-alpha
- "Emerging therapies:
- Immunostimulant: muramyl-tripeptide phosphatidyl-ethanolamine (MTP-PE) – macrophage inhibitor. Recent addition of liposomal MTP-PE in combination with adjuvant chemotherapy resulted in a statistically significant increase in overall survival versus standard combination chemotherapy.
- T-cell responses by vaccination with the anti-idiotypic antibody mimicking CD55, a complement regulatory protein expressed by many solid tumors including osteosarcoma. The use of dendritic cell vaccines to enhance cytotoxic T-cell activation is being evaluated in xenograft models.
- Small molecule therapy with inhibition of the Src kinase pathway involved in osteoclast activity. The orally available Src tyrosine kinase inhibitor AZD0530 is currently being investigated in a phase II clinical trial in osteosarcoma with pulmonary recurrence post-metastasectomy."[4]
 Diagnostic Tests/Lab Tests/Lab Values[edit | edit source]
Diagnostic Tests/Lab Tests/Lab Values[edit | edit source]
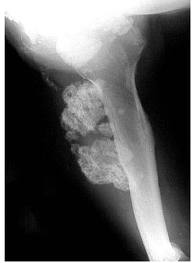
- Radiographs should be taken in any patient with prolonged and unexplained bone pain
- Magnetic resonance imaging (MRI)
- Chest CT Scan to detect pulmonary metastasis
- Nuclear Imaging to aid in the initial staging/metastatic evaluation and response to therapy
- Technetium-99 bone scans [4]
- Positron Emission Tomography (PET) Scan
- Bone Biopsy – Biopsy is the gold standard for diagnosis of bone sarcoma
- Blood Tests – Blood tests are not conclusive, but they may be helpful once a diagnosis is made to help determine the staging of cancer. High levels of alkaline phosphatase and lactate dehydrogenase (LDH) may suggest that the osteosarcoma is more advanced.[8]
Etiology/Causes [9][edit | edit source]
- The pathogenesis and etiology of osteosarcoma remains obscure
- Chemical agents such as beryllium
- Viruses such as FBJ (which contain the src-oncogene)
- Radiation
- Paget’s disease
- Electrical burn and trauma
- Genetic predisposition: hereditary retinoblastoma
Systemic Involvement[edit | edit source]
The lungs are most often the first site of metastasis and are also the most common. The articular cartilage is thought to form a natural barrier to direct tumor extension.[10]
Medical Management (current best evidence)[edit | edit source]
- Chemotherapy
- Radiation
- Surgery
- The distal femur is the most common site for osteosarcoma [10]
- Approximately 70%-80% of all lesions in this location can be safely treated by a limb-sparing resection[11]
- Amputation is reserved for those patients whose primary tumor is believed to be unresectable
- The pelvis and proximal femur are the sites of approximately 5%-10% [11]
- Hemipelvectomy is often required for pelvic tumors.[10]
- Treatment is a combination of chemotherapy and surgery
- Use of adjuvant chemotherapy increases survival from < 20% to > 65% at 5 years [11]
- Chemotherapy usually begins before any surgery
- Desired response is for > 95% tumor necrosis [11]
- In limb-sparing surgery the tumor is resected en bloc [11]
- 80% of patients can be treated with limb-sparing surgery without decreasing long-term survival rate[11]
- Continuation of chemotherapy after surgery is usually necessary
- If there is nearly complete tumor necrosis (about 99%) from preoperative chemotherapy, 5-yr survival rate is > 90%.[11]
Physical Therapy Management (current best evidence)[edit | edit source]
Osteosarcoma will be treated at large medical centers where a multi-disciplinary team including physical therapists, occupational therapists, pediatricians, surgeons, psychologists and nursing staff will help manage the patient care. In an oncology setting, physical therapists manage the patient’s musculoskeletal, neuromuscular, integumentary and cardiopulmonary rehabilitation needs.
Physical therapy interventions will consist of early postsurgical mobility training, strength and endurance restoration, pain control, and education and training of family members in helping patients with limited mobility. Physical therapists will help to correct balance and coordination impairments, make recommendations for home modifications that will enhance the patient’s independence, and educate and train the family members to assist and enable the patient to function independently. In addition, physical therapists will train the patient in stump management and training with the prostheses for those patients who undergo amputation.[12]
Rehabilitation following limb sparing procedures, rotationplasty or amputations focuses on retraining muscles, increasing strength and endurance, balance and range of motion as well as helping the patient return to school or work activities. The Functional Mobility Assessment (FMA) is a new tool for patients with lower extremity sarcoma. FMA requires the patient to perform functional mobility tasks. The FMA provides a reliable and valid measure of objective functional outcome. The use of this tool may help therapists guide the patient in returning to daily activities.[1]
Outcome measures such as the L Test of Functional Mobility is the modified version of the Timed Up and Go test designed for people with lower-limb ambutations.[13] The Amputee Mobility Predictor is an instrument to assess the ambulatory potential of lower-limb amputees with and without the use of a prosthesis.[14]
Alternative/Holistic Management (current best evidence)[edit | edit source]
Currently there is no evidence or high quality clinical study to support alternative management of osteosarcoma.
Differential Diagnosis[edit | edit source]
- Ewing’s Sarcoma
- Rhabdomyosarcoma
- Chondrosarcoma
- Chondroma
- Giant Cell Tumor
- Paget’s Disease
- OsGood-Schlatter’s Disease
Case Reports/ Case Studies[edit | edit source]
- Ayerza MA, Farfalli GL, Aponte-Tinao L, Muscolo DL. Does increased rate of limb-spraing surgery affect survival rate in osteosarcoma? Clinical Orthopaedics and Related Research. 2010; 468:11, 2854-9.
- Campanacci L, Manfrini M, Colangeli M, Ali N, Mercuri M. Long-term results in children with massive bone osteoarticular allografts of the knee for high-grade osteosarcoma. Journal of Pediatric Orthopedics. 2010; 30:8;919-27.
- Nagarajan R, Kamruzzaman A, Ness KK, Marchese VG, Sklar C, et al. Twenty years of follow-up of surviors of childhood osteosarcoma; A report from the childhood cancer survivor study. Cancer. 2011; 117:3, 625-34.
- Webber NP, Seidel M. The cutting edge. Combining advanced technologies: the Compress-Repiphysis prosthesis for pediatric limb salvage. Orthopedics. 2010;33(11)823-7.
- Wu P, Chen W, Lee O, Chen C, Huang C, Chen T. The prognosis for patients with osteosarcoma who have received prior manipulative therapy. J Bone Joint Surgery. 2010. 92:11, 1580-5.
- Paluska S. Persistent knee pain in a recreational runner. Department of Family Medicine. University of Washington, Seattle. 2002.
- Sahin H, ,Ceylan N, Bayraktarogulu S, Savas R. Cardiac metastasis of osteosarcoma: A case report.Central European Journal of Medicine. 2010; 5:551-555.
- Koob M, Durckel J, Dosh J, Entz-Werle N, Dietemann JL. Intercostal myositis misdiagnosed as osteosarcoma in a 10 year-old child.Pediatric Radiology. Springer-Verlag. 2010; 40:1, S34-S37.
- Intracortical osteosarcoma of the tibia in a 14-year-old girl. http://www.ncbi.nlm.nih.gov/pubmed/21304410
- Karacalioglu O, Ilgan S, Kuzhan O, Emer O, Ozguven M. Disseminated metastatic disease of osteosarcoma of the femur in the abdomen: unusual metastatic patter on Tc-99N MDP bone scan. Annals of Nuclear Medicine. 2006; 20:6, 437-440. http://www.jsnm.org/files/paper/anm/ams206/ANM20-6-09.pdf.
Resources
[edit | edit source]
• Merck Manual: http://www.merckmanuals.com
• National Cancer Institute: http:// www.cancer.gov
• National Institute for Health: http://www.nih.gov
• Texas Childrens Hospital: http://texaschildrenshosital.org
Recent Related Research (from Pubmed)[edit | edit source]
Failed to load RSS feed from http://eutils.ncbi.nlm.nih.gov/entrez/eutils/erss.cgi?rss_guid=129mA77YOoVgFoCszUzkYCwrlRedm-iKoDs_6SEy1wDlHNEMgg|charset=UTF-8|short|max=10: Error parsing XML for RSS
Latest developments in limb-salvage surgery in osteosarcoma http://www.ncbi.nlm.nih.gov/pubmed/21342040
Osteosarcoma: a review of diagnosis, management, and treatment strategies. http://www.ncbi.nlm.nih.gov/pubmed/21317869
Relationship between height at diagnosis and bone tumours in young people: a meta-analysis. http://www.ncbi.nlm.nih.gov/pubmed/21336591
Circulating endothelial cells and circulating endothelial precursor cells in patients with osteosarcoma http://www.ncbi.nlm.nih.gov/pubmed/21319292
MR Imaging Findings of a Primary Cardiac Osteosarcoma and Its Bone Metastasis with Histopathologic Correlation
http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3017878/?tool=pubmed
- ↑ 1.0 1.1 1.2 1.3 Goodman C, Fuller K. Pathology: Implications for the Physical Therapist. 3rd ed. St. Louis: Saunders Elsevier; 2009.
- ↑ Benayahu D, Shur I, Marom R, Meller I, Issakov J. Cellular and molecular properties associated with osteosarcoma cells. Journal of Cellular Biochemistry. 2002; 84:108-114.
- ↑ 3.0 3.1 3.2 3.3 3.4 Osteosarcoma/Malignant Fibrous Histiocytoma of Bone. National Cancer Institute. www.cancer.gov. Accessed February 18, 2011.
- ↑ 4.0 4.1 4.2 Federman N, Bernthal N, Eilber F, Tap W. The multidisciplinary management of osteosarcoma. Current Treatment Options in Oncology. 2009; 10:82-93.
- ↑ Kansara M, Thomas D. Molecular Pathogenesis of Osteosarcoma. DNA and Cell Biology. 2007; 26:1; 1-18.
- ↑ Unni K. Osteosarcoma of the bone. J Orthop Sci. The Japanese Orthopaedic Association. 1998; 3:287-294.
- ↑ Miller R. Contrasting Epidemiology of Childhood Osteosarcoma, Ewing’s Tumor, and Rhabdomyosarcoma. National Cancer Institute. 1981; 56; 9-15.
- ↑ Siegel H, Pressey J. Current concepts on the surgical and medical management of osteosarcoma. Expert Rev. Anticancer Ther. 2008; 8:8; 1257-1269.
- ↑ www.mereckmanual.com
- ↑ 10.0 10.1 10.2 Aboulafia A, Malawer M. Surgical management of pelvic and extremity osteosarcoma. Cancer Supplement. 1993; 71:10; 3358-3366.
- ↑ 11.0 11.1 11.2 11.3 11.4 11.5 11.6 www.cancer.org/Cancer/Osteosarcoma/DetailedGuide/osteosarcoma-diagnosis accessed March 11, 2011.
- ↑ Punzalan M, Hyden G. The role of physical therapy and occupational therapy in the rehabilitation of pediatric and adolescent patients with osteosarcoma. Anderson Cancer Center. Houston, TX. 2009.
- ↑ Deathe A, Miller W. The L test of functional mobility: Measurement properties of a modified version of the timed "up and go" test designed for people with lower-limb amputations.
- ↑ Cite error: Invalid
<ref>tag; no text was provided for refs namedfourteen