Basal Ganglia: Difference between revisions
No edit summary |
No edit summary |
||
| Line 14: | Line 14: | ||
The '''corpus striatum''' is the largest component of the basal ganglia'''.''' It contains the '''caudate nucleus''' and '''lenticular nuclei''', which is made up of the '''putamen''', '''globus pallidus externus''', and '''globus pallidus internus.''' The '''subthalamic nucleus''' (STN), and the '''substantia nigra''' (SN) are also components of the basal ganglia. These structures work together to promote or inhibit movement.<ref name=":1">Young CB, Sonne J. [https://www.ncbi.nlm.nih.gov/books/NBK537141/ Neuroanatomy, basal ganglia.] InStatPearls [Internet] 2018 Dec 28. StatPearls Publishing.</ref>[[File:Striatum.png|400x400px|thumb|Doral Corpus Striatum ]] | The '''corpus striatum''' is the largest component of the basal ganglia'''.''' It contains the '''caudate nucleus''' and '''lenticular nuclei''', which is made up of the '''putamen''', '''globus pallidus externus''', and '''globus pallidus internus.''' The '''subthalamic nucleus''' (STN), and the '''substantia nigra''' (SN) are also components of the basal ganglia. These structures work together to promote or inhibit movement.<ref name=":1">Young CB, Sonne J. [https://www.ncbi.nlm.nih.gov/books/NBK537141/ Neuroanatomy, basal ganglia.] InStatPearls [Internet] 2018 Dec 28. StatPearls Publishing.</ref>[[File:Striatum.png|400x400px|thumb|Doral Corpus Striatum ]] | ||
* '''Corpus Striatum''': a heterogeneous structure with a volume of around 10 cm<sup>3</sup>. It receives afferent inputs from various cortical and subcortical structures and sends outputs to different basal ganglia nuclei.<ref name=":0" /> There are two main divisions in the corpus striatum: | * '''Corpus Striatum''': a heterogeneous structure with a volume of around 10 cm<sup>3</sup>. It receives afferent inputs from various cortical and subcortical structures and sends outputs to different basal ganglia nuclei.<ref name=":0" /> There are two main divisions in the corpus striatum: | ||
** '''Dorsal Striatum''' (DS) (shown in red in image): mostly involved in the control of conscious motor movements and executive functions. The dorsal striatum consists of the caudate nucleus and the putamen. The internal capsule is a white matter nerve tract in the dorsal striatum separates the caudate nucleus from the putamen. | ** '''Dorsal Striatum''' (DS) (shown in red in the image): mostly involved in the control of conscious motor movements and executive functions. The dorsal striatum consists of the caudate nucleus and the putamen. The internal capsule is a white matter nerve tract in the dorsal striatum that separates the caudate nucleus from the putamen. | ||
** '''Ventral Striatum''': involved in the limbic functions of reward and aversion. It is made up of the nucleus accumbens and the olfactory tubercle.<ref name=":1" /> | ** '''Ventral Striatum''': involved in the limbic functions of reward and aversion. It is made up of the nucleus accumbens and the olfactory tubercle.<ref name=":1" /> | ||
* Internal and external segments of the '''Globus Pallidus''': together, the globus pallidus and putamen form the lentiform (or lenticular) nucleus.<ref>Javed N, Cascella M. [https://www.ncbi.nlm.nih.gov/books/NBK557755/ Neuroanatomy, Globus Pallidus.] [Updated 2023 Feb 20]. In: StatPearls [Internet].</ref> | * Internal and external segments of the '''Globus Pallidus''': together, the globus pallidus and putamen form the lentiform (or lenticular) nucleus.<ref>Javed N, Cascella M. [https://www.ncbi.nlm.nih.gov/books/NBK557755/ Neuroanatomy, Globus Pallidus.] [Updated 2023 Feb 20]. In: StatPearls [Internet].</ref> | ||
| Line 37: | Line 37: | ||
=== Parkinson's === | === Parkinson's === | ||
Parkinson's is the second most common neurodegenerative disorder. Its aetiology is multifactorial, with both genetic and environmental risk factors identified.<ref>Ben-Shlomo Y, Darweesh S, Llibre-Guerra J, Marras C, San Luciano M, Tanner C. The epidemiology of Parkinson's disease. The Lancet. 2024;403(10423):283-92.</ref> Parkinson's is characterised "by the progressive loss of dopaminergic neurons and the formation of Lewy bodies in the affected brain areas".<ref name=":3">Zhou, ZD, Yi LX, Wang DQ, Lim TM, Tan EK. [https://translationalneurodegeneration.biomedcentral.com/articles/10.1186/s40035-023-00378-6 Role of dopamine in the pathophysiology of Parkinson’s disease]. Transl Neurodegener. 2023;12(44). </ref> Because of the degeneration of dopaminergic neurons, there is less dopamine available in the substantia nigra and striatum, which causes various clinical signs of | [[Parkinson's]] is the second most common neurodegenerative disorder. Its aetiology is multifactorial, with both genetic and environmental risk factors identified.<ref>Ben-Shlomo Y, Darweesh S, Llibre-Guerra J, Marras C, San Luciano M, Tanner C. The epidemiology of Parkinson's disease. The Lancet. 2024;403(10423):283-92.</ref> Parkinson's is characterised "by the progressive loss of dopaminergic neurons and the formation of Lewy bodies in the affected brain areas".<ref name=":3">Zhou, ZD, Yi LX, Wang DQ, Lim TM, Tan EK. [https://translationalneurodegeneration.biomedcentral.com/articles/10.1186/s40035-023-00378-6 Role of dopamine in the pathophysiology of Parkinson’s disease]. Transl Neurodegener. 2023;12(44). </ref> Because of the degeneration of dopaminergic neurons, there is less dopamine available in the substantia nigra and striatum, which causes various clinical signs of Parkinsons's, such as:<ref name=":3" /> | ||
* tremor | * tremor | ||
| Line 44: | Line 44: | ||
* muscle rigidity | * muscle rigidity | ||
=== Huntington's Disease === | |||
[[Huntington Disease|Huntington's disease]]<ref name=":4">Matz OC, Spocter M. The Effect of Huntington's Disease on the Basal Nuclei: A Review. Cureus. 2022;14(4):e24473.</ref> is a progressive, inherited neurodegerative disease. It is associated with neuropsychiatric symptoms, movement disorders (usually chorea) and cognitive impairment / dementia.<ref name=":5">Stoker TB, Mason SL, Greenland JC, Holden ST, Santini H, Barker RA. [https://pn.bmj.com/content/practneurol/22/1/32.full.pdf Huntington's disease: diagnosis and management]. Pract Neurol. 2022 Feb;22(1):32-41. </ref><ref>Medina A, Mahjoub Y, Shaver L, Pringsheim T. [https://movementdisorders.onlinelibrary.wiley.com/doi/full/10.1002/mds.29228 Prevalence and incidence of Huntington's disease: an updated systematic review and meta-analysis]. Mov Disord. 2022 Dec;37(12):2327-35. </ref> Huntington's disease occurs in individuals with a CAG expansion in the huntingtin gene.<ref name=":5" /> There is neurodegeration in many parts of the brain, the striatum<ref name=":5" /> and cortex are particularly affected.<ref name=":6">Kim A, Lalonde K, Truesdell A, Gomes Welter P, Brocardo PS, Rosenstock TR, Gil-Mohapel J. [https://www.mdpi.com/1422-0067/22/16/8363 New avenues for the treatment of Huntington's disease]. Int J Mol Sci. 2021 Aug 4;22(16):8363. </ref> Symptoms usually start in individuals aged between 30-50 years. A key motor symptom is chorea (i.e. "brief, involuntary movements that generally affect the trunk, face, and arms"<ref name=":6" />). Chorea affects voluntary movements, ultimately affecting walking, speaking and swallowing.<ref name=":6" /> | |||
Other motor signs include:<ref name=":6" /> | |||
* bradykinesia | |||
* dystonia | |||
* hyperreflexia | |||
* decreased speed of eye saccades | |||
Cognitive impairments include:<ref name=":6" /> | |||
* decreased executive function (difficulty with attention, concentration, multi-tasking, decision making) | |||
* depression | |||
* loss of memory | |||
* decreased insight | |||
The most common psyciatric conditions are:<ref name=":6" /> | |||
* | * depression | ||
* | * irritability | ||
* | * increased impulsivity | ||
=== Hemiballism === | === Hemiballism === | ||
Hemiballism<ref name=": | Hemiballism (derives from the verb “to throw” in Greek) is a rare movement disorder that causes "high amplitude movement of an entire limb or limbs on one side of the body."<ref name=":7">Hawley JS, Weiner WJ. Hemiballismus: current concepts and review. Parkinsonism Relat Disord. 2012 Feb;18(2):125-9.</ref> This condition can be caused by a number of conditions, but acute cases are often associated with focal lesions in the contralateral basal ganglia and subthalamic nucleus lesion.<ref name=":7" /> Individuals with hemiballism tend to have a good prognosis.<ref name=":7" /> | ||
=== Tourette Syndrome === | === Tourette Syndrome === | ||
<blockquote>[[Lacuna Infarcts ( Small Vessel Disease)|Silent Cerebral Infarcts]] - of interest a 2008 study found that approx. 5% of healthy middle-aged adults have microlesions in their basal ganglia. <ref>Das RR, Seshadri S, Beiser AS, Kelly-Hayes M, Au R, Himali JJ, Kase CS, Benjamin EJ, Polak JF, O'Donnell CJ, Yoshita M. [https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2712254/ Prevalence and correlates of silent cerebral infarcts in the Framingham offspring study.] Stroke. 2008 Nov 1;39(11):2929-35.</ref></blockquote> | |||
== Physiotherapy Interventions == | == Physiotherapy Interventions == | ||
== References == | == References == | ||
<references /> | <references /> | ||
Revision as of 12:58, 25 June 2024
Introduction to the Basal Ganglia[edit | edit source]
The basal ganglia is a group of subcortical nuclei, located at the base of the forebrain,[1] that is primarily involved in motor control, as well as motor learning, executive functions and emotional behaviours. It also plays an important role in reward and reinforcement, addictive behaviours and habit formation.[2]
Basal ganglia network dysfunction leads to various movement disorders,[2] such as Parkinson's and Huntington's Disease.
Structure of the Basal Ganglia[edit | edit source]
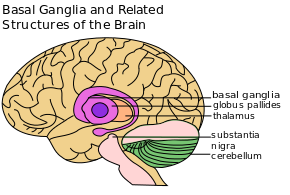
 The corpus striatum is the largest component of the basal ganglia. It contains the caudate nucleus and lenticular nuclei, which is made up of the putamen, globus pallidus externus, and globus pallidus internus. The subthalamic nucleus (STN), and the substantia nigra (SN) are also components of the basal ganglia. These structures work together to promote or inhibit movement.[3]
The corpus striatum is the largest component of the basal ganglia. It contains the caudate nucleus and lenticular nuclei, which is made up of the putamen, globus pallidus externus, and globus pallidus internus. The subthalamic nucleus (STN), and the substantia nigra (SN) are also components of the basal ganglia. These structures work together to promote or inhibit movement.[3]
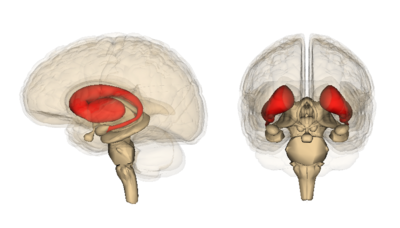
- Corpus Striatum: a heterogeneous structure with a volume of around 10 cm3. It receives afferent inputs from various cortical and subcortical structures and sends outputs to different basal ganglia nuclei.[2] There are two main divisions in the corpus striatum:
- Dorsal Striatum (DS) (shown in red in the image): mostly involved in the control of conscious motor movements and executive functions. The dorsal striatum consists of the caudate nucleus and the putamen. The internal capsule is a white matter nerve tract in the dorsal striatum that separates the caudate nucleus from the putamen.
- Ventral Striatum: involved in the limbic functions of reward and aversion. It is made up of the nucleus accumbens and the olfactory tubercle.[3]
- Internal and external segments of the Globus Pallidus: together, the globus pallidus and putamen form the lentiform (or lenticular) nucleus.[4]
- Subthalamic Nucleus (STN): a lens-shaped cell group that makes up a large part of the subthalamus.[5]
- Substantia Nigra (SN) - (which means "black substance" in Latin): while the substantia nigra is located in the midbrain, it is considered part of the basal ganglia. This long nucleus has two parts: 1) pars compacta and 2) pars reticulata. Degeneration of the pars compacta decreases the amount of available dopamine.[6]
The two optional videos below provide further information on the structure and function of the basal ganglia:
Function of the Basal Ganglia[edit | edit source]
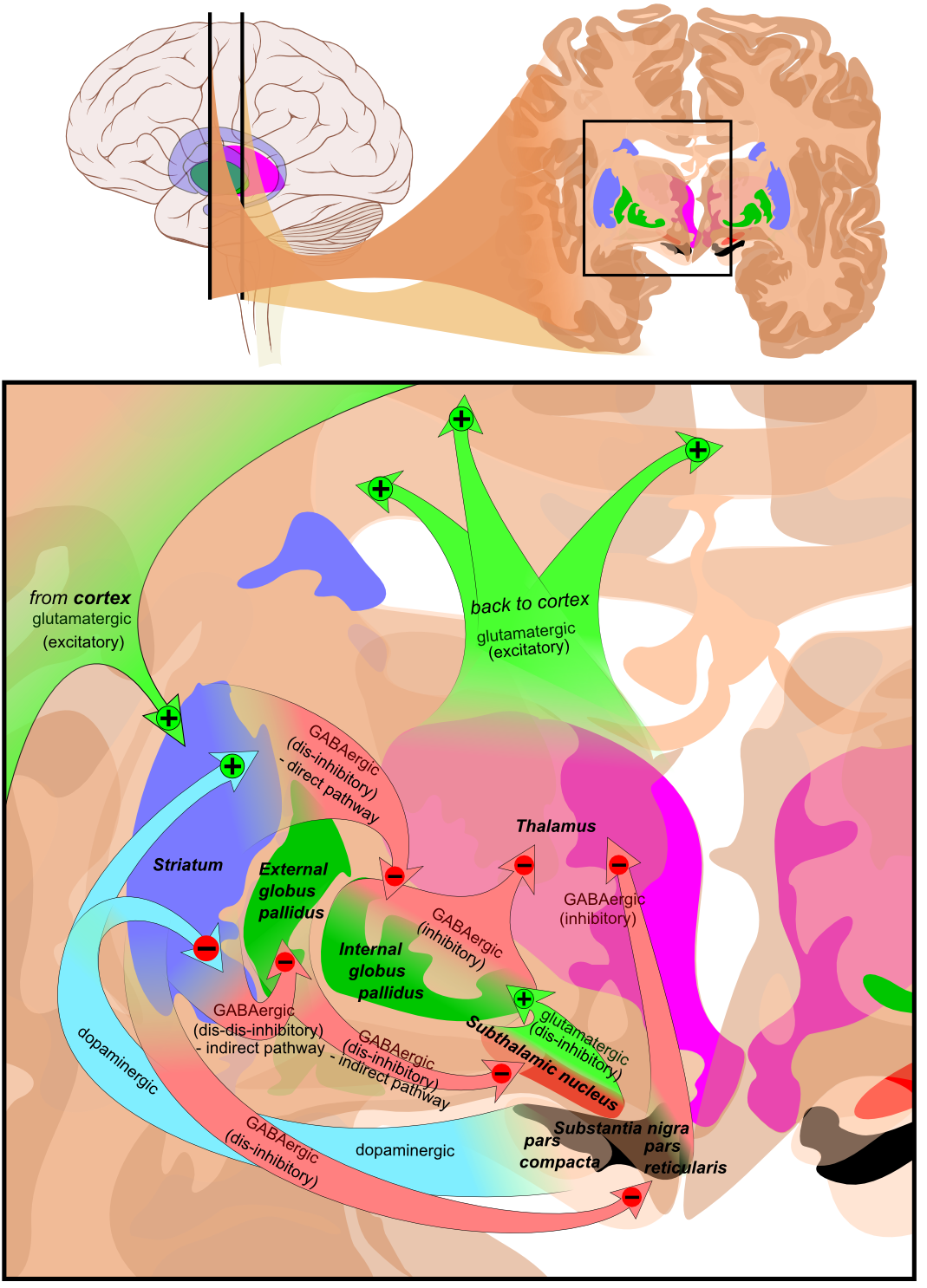
The classical basal ganglia model suggested that "information flows through the basal ganglia back to the cortex through two pathways with opposing effects for the proper execution of movement."[9] In this "loop", cortical input is sent to the basal ganglia, where it is modified and then sent back to the cortex. As a result of this feedback, motor activity is either facilitated or inhibited.[9]
This model has been reviewed and updated. It is now believed that the basal ganglia has "multiple parallel loops and re-entering circuits whereby motor, associative, and limbic territories are engaged mainly in the control of movement, behaviour, and emotions."[9]
The following image illustrates various excitatory and inhibitory pathways from the basal ganglia to the cortex.[2]
Pathophysiology[edit | edit source]
Dysfunction of the basal ganglia is associated with specific movement disorders, and can cause issues such as (1) tremor, (2) involuntary muscle movements, (3) abnormal increase in tone, (4) difficulty initiating movements, and (5) abnormal posture. The following sections discuss movement disorders associated with basal ganglia dysfunction.[3]
Parkinson's[edit | edit source]
Parkinson's is the second most common neurodegenerative disorder. Its aetiology is multifactorial, with both genetic and environmental risk factors identified.[10] Parkinson's is characterised "by the progressive loss of dopaminergic neurons and the formation of Lewy bodies in the affected brain areas".[11] Because of the degeneration of dopaminergic neurons, there is less dopamine available in the substantia nigra and striatum, which causes various clinical signs of Parkinsons's, such as:[11]
- tremor
- postural instability
- bradykinesia
- muscle rigidity
Huntington's Disease[edit | edit source]
Huntington's disease[12] is a progressive, inherited neurodegerative disease. It is associated with neuropsychiatric symptoms, movement disorders (usually chorea) and cognitive impairment / dementia.[13][14] Huntington's disease occurs in individuals with a CAG expansion in the huntingtin gene.[13] There is neurodegeration in many parts of the brain, the striatum[13] and cortex are particularly affected.[15] Symptoms usually start in individuals aged between 30-50 years. A key motor symptom is chorea (i.e. "brief, involuntary movements that generally affect the trunk, face, and arms"[15]). Chorea affects voluntary movements, ultimately affecting walking, speaking and swallowing.[15]
Other motor signs include:[15]
- bradykinesia
- dystonia
- hyperreflexia
- decreased speed of eye saccades
Cognitive impairments include:[15]
- decreased executive function (difficulty with attention, concentration, multi-tasking, decision making)
- depression
- loss of memory
- decreased insight
The most common psyciatric conditions are:[15]
- depression
- irritability
- increased impulsivity
Hemiballism[edit | edit source]
Hemiballism (derives from the verb “to throw” in Greek) is a rare movement disorder that causes "high amplitude movement of an entire limb or limbs on one side of the body."[16] This condition can be caused by a number of conditions, but acute cases are often associated with focal lesions in the contralateral basal ganglia and subthalamic nucleus lesion.[16] Individuals with hemiballism tend to have a good prognosis.[16]
Tourette Syndrome[edit | edit source]
Silent Cerebral Infarcts - of interest a 2008 study found that approx. 5% of healthy middle-aged adults have microlesions in their basal ganglia. [17]
Physiotherapy Interventions[edit | edit source]
References[edit | edit source]
- ↑ Yanagisawa N. Functions and dysfunctions of the basal ganglia in humans. Proc Jpn Acad Ser B Phys Biol Sci. 2018;94(7):275-304.
- ↑ 2.0 2.1 2.2 2.3 Lanciego JL, Luquin N, Obeso JA. Functional neuroanatomy of the basal ganglia. Cold Spring Harbor perspectives in medicine. 2012 Dec 1;2(12):a009621.
- ↑ 3.0 3.1 3.2 Young CB, Sonne J. Neuroanatomy, basal ganglia. InStatPearls [Internet] 2018 Dec 28. StatPearls Publishing.
- ↑ Javed N, Cascella M. Neuroanatomy, Globus Pallidus. [Updated 2023 Feb 20]. In: StatPearls [Internet].
- ↑ Basinger H, Joseph J. Neuroanatomy, Subthalamic Nucleus. [Updated 2022 Oct 31]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK559002/
- ↑ Jacobs LK, Sapers BL. Neurological Disease. InPerioperative Medicine 2011 (pp. 343-359). Springer, London.
- ↑ Neuroscientifically Challenged Basal ganglia . Available from: https://www.youtube.com/watch?v=OD2KPSGZ1No [last accessed 14/01/2020]
- ↑ Armando Hasudungan. The Basal Ganglia Clinical Anatomy. Available from: https://www.youtube.com/watch?v=mNc-6q6YAAw [last accessed 05/01/2024]
- ↑ 9.0 9.1 9.2 Lanciego JL, Luquin N, Obeso JA. Functional neuroanatomy of the basal ganglia. Cold Spring Harbor perspectives in medicine. 2012 Dec 1;2(12):a009621.
- ↑ Ben-Shlomo Y, Darweesh S, Llibre-Guerra J, Marras C, San Luciano M, Tanner C. The epidemiology of Parkinson's disease. The Lancet. 2024;403(10423):283-92.
- ↑ 11.0 11.1 Zhou, ZD, Yi LX, Wang DQ, Lim TM, Tan EK. Role of dopamine in the pathophysiology of Parkinson’s disease. Transl Neurodegener. 2023;12(44).
- ↑ Matz OC, Spocter M. The Effect of Huntington's Disease on the Basal Nuclei: A Review. Cureus. 2022;14(4):e24473.
- ↑ 13.0 13.1 13.2 Stoker TB, Mason SL, Greenland JC, Holden ST, Santini H, Barker RA. Huntington's disease: diagnosis and management. Pract Neurol. 2022 Feb;22(1):32-41.
- ↑ Medina A, Mahjoub Y, Shaver L, Pringsheim T. Prevalence and incidence of Huntington's disease: an updated systematic review and meta-analysis. Mov Disord. 2022 Dec;37(12):2327-35.
- ↑ 15.0 15.1 15.2 15.3 15.4 15.5 Kim A, Lalonde K, Truesdell A, Gomes Welter P, Brocardo PS, Rosenstock TR, Gil-Mohapel J. New avenues for the treatment of Huntington's disease. Int J Mol Sci. 2021 Aug 4;22(16):8363.
- ↑ 16.0 16.1 16.2 Hawley JS, Weiner WJ. Hemiballismus: current concepts and review. Parkinsonism Relat Disord. 2012 Feb;18(2):125-9.
- ↑ Das RR, Seshadri S, Beiser AS, Kelly-Hayes M, Au R, Himali JJ, Kase CS, Benjamin EJ, Polak JF, O'Donnell CJ, Yoshita M. Prevalence and correlates of silent cerebral infarcts in the Framingham offspring study. Stroke. 2008 Nov 1;39(11):2929-35.