Palliative Care Competence Framework for Physiotherapists: Difference between revisions
No edit summary |
No edit summary |
||
| Line 293: | Line 293: | ||
If patients present with any of the above conditions, a decision regarding different methods of treatment will need to be discussed. | If patients present with any of the above conditions, a decision regarding different methods of treatment will need to be discussed. | ||
< | <br> | ||
<u>'''PICTURE 1 - treatment decision'''</u> | <u>'''PICTURE 1 - treatment decision'''</u> | ||
'''<u></u>''' | '''<u></u>''' | ||
'''<u>MLLB</u>: '''<br>MLLB generally consists of a tubular lining, digit bandages, padding and layers of inelastic bandages. Due to the rapid change in limb shape and oedema reduction during the first 1-2 weeks bandages need to be changed daily to maintain the pressure (Williams and Keller 2005). | '''<u>MLLB</u>: '''<br>MLLB generally consists of a tubular lining, digit bandages, padding and layers of inelastic bandages. Due to the rapid change in limb shape and oedema reduction during the first 1-2 weeks bandages need to be changed daily to maintain the pressure (Williams and Keller 2005). | ||
| Line 303: | Line 303: | ||
The pressure applied through bandages is based on Lapase’s Law (Urgo Medical 2009) – | The pressure applied through bandages is based on Lapase’s Law (Urgo Medical 2009) – | ||
< | <br> | ||
<u>'''PICTURE 2 - equation'''</u> | <u>'''PICTURE 2 - equation'''</u> | ||
< | <br> | ||
P = pressure under the bandage (in mmHg), <br>T = bandage tension (Kgf)<br>N = number of layers <br>C = limb circumference (cm)<br>W = bandage width (cm) | P = pressure under the bandage (in mmHg), <br>T = bandage tension (Kgf)<br>N = number of layers <br>C = limb circumference (cm)<br>W = bandage width (cm) | ||
| Line 315: | Line 315: | ||
As with most treatments, MLLB can be adapted to suit the patient needs by either adjusting pressure, frequency of reapplication, bulk of bandage and type of bandage (Lymphoedema Framework 2006). If pressure is not applied correctly venous and lymphatic flow can be compromised, therefore the proximal movement of fluid is reduced and swelling may present in the extremities (Williams and Keller 2005). When applied to the lower limb, care must be taken to ensure that the patient is still able to wear shoes during treatment as normal gait pattern is encouraged to maintain an effective calf and foot muscle pump (Williams and Keller 2005). | As with most treatments, MLLB can be adapted to suit the patient needs by either adjusting pressure, frequency of reapplication, bulk of bandage and type of bandage (Lymphoedema Framework 2006). If pressure is not applied correctly venous and lymphatic flow can be compromised, therefore the proximal movement of fluid is reduced and swelling may present in the extremities (Williams and Keller 2005). When applied to the lower limb, care must be taken to ensure that the patient is still able to wear shoes during treatment as normal gait pattern is encouraged to maintain an effective calf and foot muscle pump (Williams and Keller 2005). | ||
<br> '''Bandaging a lymphoedema arm: (approx. 10 mins)'''<br> | |||
''' | '''[https://www.youtube.com/watch?v=uSBwkGWUcHA www.youtube.com/watch]''' | ||
''' | <u>'''YOUTUBE VIDEO 1'''</u> | ||
< | <br> '''Bandaging a lymphoedema leg: (approx. 10 mins)''' | ||
[https://www.youtube.com/watch?v=-jC6LzOlI1w www.youtube.com/watch] | |||
'''Bandaging a lymphoedema leg: (approx. 10 mins)''' | |||
<u>'''YOUTUBE VIDEO 2'''</u> | <u>'''YOUTUBE VIDEO 2'''</u> | ||
<br> | |||
Inelastic and elastic bandages have been discussed, however more recently, a new bandaging system known as Coban 2 has been developed which can be used an alternative to MLLB. Coban 2 consists of a comfort layer and a compression layer that cohesively bond together (Lamprou et al. 2011). This eliminates the need for a thick padding layer that MLLB requires, resulting in a much less bulky appearance allowing patient’s more mobility and freedom.<br> | Inelastic and elastic bandages have been discussed, however more recently, a new bandaging system known as Coban 2 has been developed which can be used an alternative to MLLB. Coban 2 consists of a comfort layer and a compression layer that cohesively bond together (Lamprou et al. 2011). This eliminates the need for a thick padding layer that MLLB requires, resulting in a much less bulky appearance allowing patient’s more mobility and freedom.<br> | ||
| Line 339: | Line 338: | ||
A multicentre randomised controlled trial with 82 participants (Moffatt et al. 2011) investigating the frequency of application of Coban 2. Results found constant therapeutic effect was maintained when bandages were reapplied every four days. Compared with MLLB, which requires reapplication daily at certain stages of treatment, the Coban 2 bandages allow patients to have more freedom and independence.<br> | A multicentre randomised controlled trial with 82 participants (Moffatt et al. 2011) investigating the frequency of application of Coban 2. Results found constant therapeutic effect was maintained when bandages were reapplied every four days. Compared with MLLB, which requires reapplication daily at certain stages of treatment, the Coban 2 bandages allow patients to have more freedom and independence.<br> | ||
<br> '''Coban 2 bandaging: (approx. 15 mins) '''<br><u>'''YOUTUBE VIDEO 3'''</u> | |||
<u>'''[https://www.youtube.com/watch?v=5iGA9tCyFyA www.youtube.com/watch]'''</u><br> | |||
As discussed, a number of trials have evaluated the effects of compression bandaging for lymphoedema, which have found positive results. The development of Coban 2 bandages is encouraging, however this still requires larger scale trials to fully evaluate the efficacy.<br> | |||
<u</u><u</u>As discussed, a number of trials have evaluated the effects of compression bandaging for lymphoedema, which have found positive results. The development of Coban 2 bandages is encouraging, however this still requires larger scale trials to fully evaluate the efficacy.<br> | |||
Now that compression bandaging has been considered, this wiki will now move on to discuss the use of compression garments which are used for the long-term management of limb shape and swelling (Lymphoedema Framework 2006).<br> | Now that compression bandaging has been considered, this wiki will now move on to discuss the use of compression garments which are used for the long-term management of limb shape and swelling (Lymphoedema Framework 2006).<br> | ||
| Line 356: | Line 356: | ||
Once the progression from bandages to garments has been made, swelling and other symptoms must be monitored. If swelling is not controlled within the first three months of wearing compression garments, clinician and patient should consider further intensive therapy using MLLB (Moffatt et al 2005).<br>Measurement for hosiery will be undertaken by a qualified health professional, which may be a physiotherapist | Once the progression from bandages to garments has been made, swelling and other symptoms must be monitored. If swelling is not controlled within the first three months of wearing compression garments, clinician and patient should consider further intensive therapy using MLLB (Moffatt et al 2005).<br>Measurement for hosiery will be undertaken by a qualified health professional, which may be a physiotherapist | ||
<br> | |||
<u>'''PICTURE 3 - meausuring for compression garments'''</u><br> | <u>'''PICTURE 3 - meausuring for compression garments'''</u><br> | ||
< | <br> | ||
Garments are either constructed as a flat knit or a round knit. Flat knit knitted as a flat garment then joined at the seams, these are generally thicker and firmer. Round knitted garments are viewed as more aesthetically pleasing, as they are thinner than flat knit and are continuously knitted cylindrically without any seams (Lymphoedema Framework 2006).<br> | Garments are either constructed as a flat knit or a round knit. Flat knit knitted as a flat garment then joined at the seams, these are generally thicker and firmer. Round knitted garments are viewed as more aesthetically pleasing, as they are thinner than flat knit and are continuously knitted cylindrically without any seams (Lymphoedema Framework 2006).<br> | ||
| Line 367: | Line 367: | ||
As well as limb compression garments, if patients have trunk or breast lymphoedema garments or specialised bras can be provided (Lymphoedema Framework 2006). | As well as limb compression garments, if patients have trunk or breast lymphoedema garments or specialised bras can be provided (Lymphoedema Framework 2006). | ||
'''<u></u>'''<u>'''Activity'''</u> | '''<u></u>'''<u>'''Activity'''</u> | ||
Revision as of 23:31, 17 January 2016
Original Editor - Your name will be added here if you created the original content for this page.
Top Contributors - Charlotte Kay, Yasmin Natasha Milne, Emma Clare Sneddon, Sarah Brennan, Lauren Lopez, Kim Jackson, 127.0.0.1, Jane Hislop, Admin, Evan Thomas and Lucinda hampton
</div>
Introduction and Learning Outcomes[edit | edit source]
Welcome to this online learning resource focussing on “The role of the Physiotherapist in Palliative Care for people with Lymphoedema”. This has been designed by a group of fourth year Physiotherapy students from Queen Margaret University as part of the “Contemporary and Emerging Issues in Physiotherapy” module.
Aims
The aim of this wiki is to present a learning resource for final year physiotherapy students and new graduates to develop their knowledge and understanding of:
- The role of a physiotherapist in the management of people with lymphoedema
- The implications for physiotherapy practice when managing people with lymphoedema in a palliative care setting
Learning Outcomes
By the end of this wiki you should be able to:
- Discuss the background of lymphoedema and describe the key presenting symptoms
- Discuss the physiotherapist’s role in the management of people with lymphoedema
- Critically reflect on the physiotherapist’s approach in the management of people with palliative care needs who have lymphoedema
- Critically appraise and synthesise the literature relating to the end of life care for a person with lymphoedema
Overview of Lymphoedema
[edit | edit source]
Introduction[edit | edit source]
This section of the wiki will aim to provide an overview of:
- The pathophysiology of lymphoedema
- The two types of lymphoedema - primary and secondary
- The four stages of the condition
- Clinical features (physical and psychological) those with the condition may present
- Two of the main leading causes related to lymphoedema - cancer and infection
- Epidemiology regarding the condition
Lymphatic System[edit | edit source]
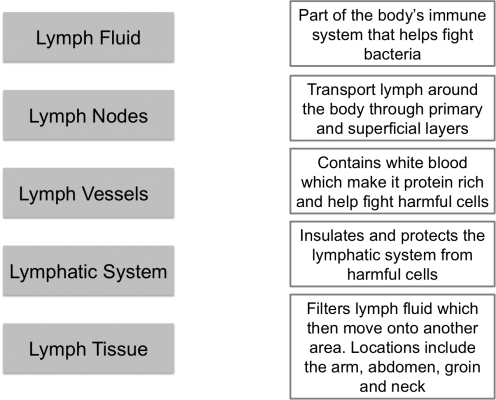
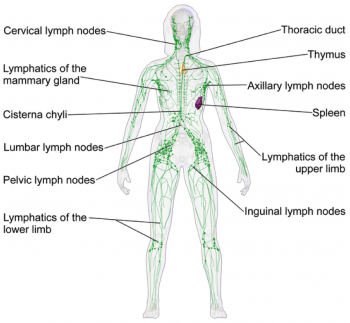
The lymphatic system is part of the body's immune system that plays a role in fighting harmful cells, for example, bacteria. It consists of lymph fluid, lymph nodes, lymph vessels and lymph tissue.
Lymph tissue insulates and protects the lymphatic system from damaging cells. Lymph fluid contains infection-fighting white blood cells. It circulates throughout the lymphatic system and is formed when interstitial fluid is collected through lymph channels (vessels, ducts and capillaries).
Lymph is primarily made up of a white watery substance:
PICTURE OF LYMPH COMPOSITION
The main lymphatic functions:
1. Helps immune system respond to the body
2. Redistribution of fluid in the body
3. Lymph carries proteins, solids, and liquids away from tissue space
a. Remove waste products from interstitial space (between all body tissue) (Seifter et al 2005)
b. Bacteria, toxins and foreign bodies are removed from tissues
4. Controls the flow of large molecules around the body (National Lymphoedema Network 2013)
5. Controls tissue fluid homeostatsis (Ridner 2013) to maintain the structure and functional aspects of tissue.
The flow of lymph fluid is unidirectional, towards the heart to provide cells with oxygen. It is protein-rich and fights abnormal cells due to its white cell content (Cancer Research UK 2014).
There are approximately 600 to 700 lymph nodes located around the body, specifically under the arm, in the abdomen, groin and neck. (Hampton 2015). Their role involves transporting lymph fluid around the body using specific lymph channels located on left and right sides. The fluid then passes through superficial primary lymph vessels (that drain the skin) and is emptied into deep secondary lymph vessels (which also contains drainage from internal organs). Adequate flow of lymph fluid is dependant on muscle contractions and an efficient respiratory system, for example, exercise (Kerchner et al 2008).
The lymphatic system can become blocked from localised fluid retention and tissue swelling within the body – known as Lymphoedema. It occurs when the function of the lymphatic system is compromised in some way. Lymph pathways are unable to exchange nutrients effectively within the interstitial spaces, causing a build up of excess fluid. Upper and lower extremities are affected depending on which area of the body is damaged. The cause of onset determines whether the affected person has either primary or secondary lymphoedema.
Activity[edit | edit source]
Take 10 mins to complete the activity below. Using the information provdied so far, match the different components of the lymphatic system with their definitions:
Types of Lymphoedema[edit | edit source]
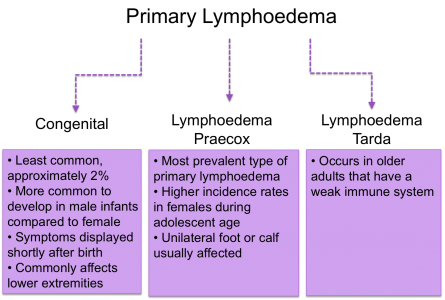
Primary
Approximetaly 1 in 6000 people develop primary lymphoedema. This form of lymphoedema isn’t inherited through family history and wouldn’t be passed onto future generations. However, people can develop primary lymphoedema in relation to other genetic and congenital abnormalities (Lymphoedema Support Group 2003).
There are three classifications depending on the onset of symptoms:
Secondary
Secondary lymphoedema is more common than the primary form. The lymphatic system is damaged due to an external cause compromising the function of the lymph nodes. Consequently, swelling accumulates in the affected part of the body.
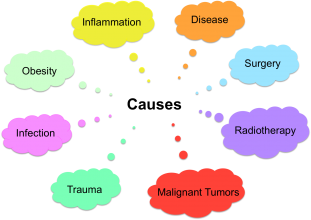
Causes of secondary lymhoedema include (NHS Choices 2014):
- Surgery (cancer and non-cancer related) – increases the risk of disturbing the function of lymphatic pathways
- Radiotherapy - destroys cancerous tissue but can also damage healthly lymph nodes
- Infections – contributes to increased swelling in the affected area
- Inflammation – contributes to excess fluid build up in the affected area
- Obesity – increased the pressure on the lymphatic system that could ultimately damage lymph nodes
- Disease (venous or joint)
The most common causes of secondary lymphoedema are cancer and non-cancer related surgery. Although these treatments have a number benefits, their outcomes can lead to disruption of the lymphatic system.
In developing countries the most common cause of secondary lymphoedema is filiariasis, a parasitic infection with filarial worms (WHO 2016). In more developed countries malignancy is the main root of cause for acquiring secondary lymphoedema (Kerchner 2008).
Causes of upper limb lymphoedema include (The Lymphoedema Support Network year):
- Trauma or injury – removal of lymph nodes during breast cancer surgery, upper body radiotherapy, burns, and scarring
- Cancer that has spread to the upper body compromising the function of the lymph nodes
- Following deep vein thrombosis (DVT) or high doses of intravenous (IV) drugs
- Reduced upper limb mobility as a result of an illness, for example, multiple sclerosis or stroke
Stages Of Lymphoedema[edit | edit source]
(breastcancer.org 2015)
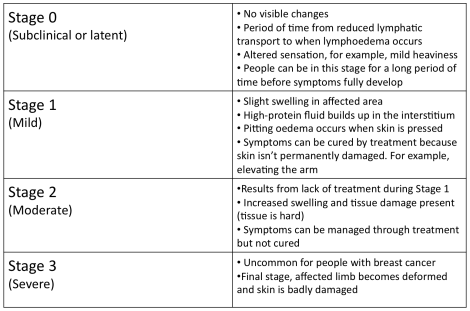
There are 4 Stages of lymphoedema:
Lymphoedema is a chronic and incurable condition so treatment strategies focus on reducing disease progression, for example, swelling management and infection prevention.
Clinical Features
[edit | edit source]
There are both physical and psychological effective of the chronic condition. Early diagnosis is vital to ensure the correct treatment is chosen.
Physical changes
- In the early stages pitting oedema occurs where the skin is pressed leaving an indent in the swelling. Elevating the arm creates a draining effect to reduce swelling
- Limbs can feel heavy and achy
- There is altered sensation, for example, pins and needles
- Reduced mobility and range of movement of the affected limb/s
- Pain and joint discomfort
- Skin changes, for example redness and increased temperature
- Nail discoloration (Lyons and Modarai 2013)
- Hyperkeratosis (thickening of the skin) and lymphangiectasia (dilated superficial lymph vessels) (The Lymphoedema Support Network 2015)
(Cancer Research UK 2014; McCallin et al 2005)
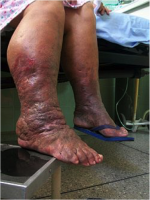
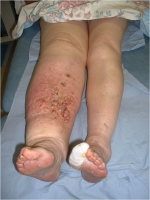
When the condition affects the lower extremities, over time the affected person’s gait pattern is altered, leading to a higher risk of disability.
Psychological Effects
There are psychological effects associated with the condition as a result of changes to body image.
- Swelling and weight gain impact physical appearance that can affect one’s perception of how they look, consequently decreasing their self-confidence (Harmer 2009; McCallin et al 2005)
- People commonly detach themselves from social events with family and friends leading to social isolation (Ridner 2009)
- Disturbed sleeping pattern
- Some people may feel they have a lack of support
- Financial concerns as a consequence of treatment cost and potential job loss/change (Ridner 2009)
Mason et al (2008) conducted a systematic review of literature that looked at the psychosocial aspects related lymphoedema. It was found that people with the condition experience anger, depression, anxiety and relationship issues. People can feel embarrassed having to wear different clothes due to compression bandaging, swelling and weight gain. Ultimately, there is an overall decrease in quality of life (QoL) from reduced social and leisure activities. The study concluded more research is required that focuses on improving specific psychosocial issues rather targeting QoL to reslove issues such as anger and depression.
Another study looked at the incidence, cost of treatment and complications of lymphoedema following breast cancer treatment. It concluded that 10% of the 1877 participant showed signs of lymphoedema 2 years after breast cancer treatment. A complication of the condition was the high medical costs for treatment. This lead to increased length of stay in hospital and ultimately reduced the patient’s quality of life (Ridner 2009).
It is important for health professionals to recognise and fully understand the psychological and psychosocial implications for each individual patient to ensure person-centred care is provided. Communication and appropriate referrals to other health professionals is important in overall management of the condition, for example social workers and psychologists.
Activity[edit | edit source]
This activity will take approximately 15 minutes to complete.
Relfect on your learning regarding the different stages of lymphoedema and think about how a person's body image will change as they progress through each stage. Other resources such as the internet, books and journals will provide additional information and images to help enhance your understanding when completing this activity.
Leading Causes[edit | edit source]
Cancer
Lymphoedema following breast cancer surgery is the highest overlooked cause of secondary lymphoedema. Harmer (2009) states approximately 20% of people will acquire lymphoedema after receiving this treatment. The procedure involves removing one or more lymph nodes located under the arm. Consequently, the remaining lymph nodes are put under strain and unable to work properly resulting in a build up of excessive fluid in the affected area (breastcancer.org 2015). Cancer Research UK (2014) discusses the vicious cycle between cancer and the body’s immune system. Cancerous cells are destroyed by the immune system and treatments for cancer. However, the condition can weaken the immune system if lymph nodes are blocked by cancerous tissue and unable to function properly.
A combination of surgery and radiotherapy treatment leads to a higher risk of acquiring secondary lymphoedema. Radiation therapy aims to stop cancer from coming back by using high-radiation energy to destroy cancerous cells (National Cancer Institute 2010). It either occurs before surgery to reduce the size of a tumour, or after surgery to abolish the remainder of the tumour. Lymphoedema can occur as a result of this treatment when the function of the lymphatic system has been comprised and fluid isn’t drained away (NHS Choices 2014).
Infection
Infection is a key issue commonly related to lymphoedema. It either results from swelling or causes it to develop (Hampton 2015). Lymph nodes help fight infections but when they are damaged infections can develop quicker. The Lymphoedema Support Network (2010) defines cellulitis as “acute spreading inflammation of the skin and subcutaneous tissue”. It causes the skin to become warm, red, swollen and painful with onset either sudden or progressing over a few hours. If the lymph tissue is damaged there is added strain on the lymphatic system. In an infected limb, the inflammatory process cause attracts fluid causing an increase in swelling. Consequently, lymphoedema is exacerbated during this period of infection (McGilvray year).
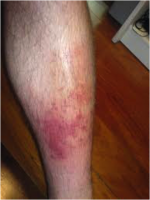
75-90% of cellulitis occurs in the lower body and is caused by bacteria entering inflamed or broken skin. A study found that the condition was responsible for 3% of admissions in a UK hospital (Al-Niaimi and Cox, 2009). This common occurrence puts financial strain on the NHS; therefore, infection prevention is a large part of lymphoedema treatment. There is a strong link between leg cellulitis and lymphoedema, where progression of the condition can lead to ulceration and septicaemia. Each cellulitis episode exacerbates secondary lymphoedema, which in turn increases the risk for a further infection episode. A Cochrane review found that a quarter of lymphoedema patients would acquire cellulitis (Al-Niaimi and Cox, 2009).
Above: Cellulitis in a lower limb
Epidemiology[edit | edit source]
Epidemiology looks at why diseases or conditions develop in different societies and how common the occurnace is. The information gathered is then used to improve current and future healthcare in disease management (BMJ 2016). Below provides an insight into some of the epidemiology regarding lymphoedema:
- Lymphoedema occurs in approximately 240,000 people in the UK with older adults more susceptible than the younger population (Nazarko 2015)
- Within 3 years after cancer surgery 80% of people will develop secondary lymphoedema
- Another study concluded 1 in 5 patients will develop lymphoedema after 6 months post breast cancer surgery (Harmer 2009)
- One study looked at a population of 600,000 people over the age of 65 and concluded 1 in 200 people had chronic lymphoedema. 50% of these people had a reduced quality of life and hospital care cost the NHS £2300 (Hampton 2015)
- Rockson and Rivera (2008) mention that 1.15 in 100,000 people under the age of 20 will acquire primary lymphoedema
- One study surveyed 308 centres (2743 people) in Spain and found that 36.8% suffered from primary lymphoedema. Of the 36.8%, 2% had acquired it at birth, 30% during adolescence and 68% were older adults (Williams et al 2005)
- One study found that out of 287 breast cancer survivors, 48% had upper limb swelling at least once and 34% had clinical symptoms of lymphoedema 6 years post treatment (Ridner 2013)
Activity[edit | edit source]
Now that you have completed this section of the wiki module. Take approximately 15 minutes to complete the following quiz
Conclusion[edit | edit source]
This section aimed to provide an insight into the background of lymphoedema to form a base of knowledge that will be futher developed throughout the rest of this wiki. It is important to understand the pathophysiology and presenting symtoms because this ensures the correct treatment strategy is chosen.
By the end of this section you should be able to:
- Discuss the background of lymphoedema and describe the key presenting symptoms
The next section of the wiki focuses on discussing the physiotherapists role in managing lymphoedema and critically anlayses a range of available. Before progressing onto this section, please ensure you have taken time to complete all the activities above.
Treatment and Management of Lymphoedema[edit | edit source]
Introduction[edit | edit source]
The Lymphoedema Framework (2006) states that the treatment of people with lymphoedema should be specifically tailored based on the site, severity and complexity as well as their psychosocial situation. Furthermore the success of treatment does not solely rest on the therapist, patients and carers must play an active role from an early stage.
Alongside the physical difficulties that people with lymphoedema face, emotional and social implications may also arise. Evidence has suggested that through specific management and targeting of the physical symptoms, the psychosocial issues can be reduced to enhance the individual’s quality of life (Kim and Park 2008).
Decongestive lymphatic therapy (DLT) is viewed as the gold standard of care for lymphoedema (Chang and Cormier 2013). Kim and Park (2008) found this treatment approach effective and significantly reduced the percentage excess limb volume as well as improving quality of life.
Decongestive lymphatic therapy encompasses four main components (Todd 2009);
- Compression
- Skin care
- Exercise
- Massage
The management of lymphoedema is split into intensive and maintenance stages, both of which have very different approaches. The goals during the intensive stage of therapy are to reduce and control the swelling, maintain skin quality and educate the patient in order for them to reach a stage where they are ready to progress into the maintenance phase of treatment (Korpan et al. 2011). This is achieved through a number of approaches aiming to reduce the load and decongest the lymphatic system, stimulate the function and encourage drainage through various routes (Lymphoedema Framework 2006). Once swelling is bought under control patients will progress to the maintenance stage. During this stage people with lymphoedema are educated to self manage their condition and will be reviewed less frequently by a specialist.
Over the next four sections of this wiki you will learn more about each of the components of DLT.
Activity
Take 5 minutes -
Can you...
- Think of any psychological issues that you may encounter if treating a patient with lymphoedema?
- Name the four cornerstones of lymphoedema treatment?
- List the goals during intensive therapy?
By this stage you should be able to identify the four cornerstones of DLT and state the general aims of the intensive and maintenance stages of treatment.
Over the next section this wiki will discuss the four components of DLT in detail with reference to the supporting evidence.
Compression Therapy[edit | edit source]
Compression therapy consists of two main methods – multilayer lymphoedema bandaging (MLLB) and compression garments. Overall, compression therapy increases lymphatic drainage, reduces capillary function, promotes fluid movement to less compressed areas of the body and improves the action of the venous pump (Cooper 2015). Furthermore, bandaging aims to improve the shape of the limb, soften fibrosclerotic tissue, support and improve skin condition and manage symptoms (Foldi et al. 2005; Williams and Keller 2005).
Once no further benefit is being obtained from compression bandaging during the intensive phase, patients should be managed by compression garments for long-term maintenance (Lymphoedema Framework 2012). However MLLB may also be used as part of long-term management if compression garments are not suitable (Moffatt et al. 2005).
The combined treatment of bandaging followed by compression garments has been found to yield better results for reduction of moderate to severe lymphoedema compared with bandaging alone. This benefit was observed as being maintained for at least six months (Badger et al. 2000), hence this is the recommended course of treatment for people with lymphoedema (Lymphoedema Framework 2012).
Indications for compression therapy as stated by the Lymphoedema Framework (2006) are:
- Fragile, damaged or ulcerated skin
- Distorted limb shape
- Limb too large for compression garments
- Areas of tissue thickening
- Lymphorrhoea
- Lymphangiectasia
- Pronounced skin folds
Not all patients will be suitable for compression therapy and this decision needs to be made by patient and clinician. There are a number of contraindications stated below (Lymphoedema Framework 2006):
- Severe arterial insufficiency
- Uncontrolled heart failure
- Severe peripheral neuropathy
If patients present with any of the above conditions, a decision regarding different methods of treatment will need to be discussed.
PICTURE 1 - treatment decision
MLLB:
MLLB generally consists of a tubular lining, digit bandages, padding and layers of inelastic bandages. Due to the rapid change in limb shape and oedema reduction during the first 1-2 weeks bandages need to be changed daily to maintain the pressure (Williams and Keller 2005).
The pressure applied through bandages is based on Lapase’s Law (Urgo Medical 2009) –
PICTURE 2 - equation
P = pressure under the bandage (in mmHg),
T = bandage tension (Kgf)
N = number of layers
C = limb circumference (cm)
W = bandage width (cm)
Bandages used are inelastic which result in high and low pressures exerted during movement and rest respectively (Moffatt et al. 2005). Elastic bandages produce less variation of pressure; these may be indicated if patients are immobile, have venous ulceration, lymphatic or venous disease or if the expected time of application is longer than normal (Lymphoedema Framework 2006).
As with most treatments, MLLB can be adapted to suit the patient needs by either adjusting pressure, frequency of reapplication, bulk of bandage and type of bandage (Lymphoedema Framework 2006). If pressure is not applied correctly venous and lymphatic flow can be compromised, therefore the proximal movement of fluid is reduced and swelling may present in the extremities (Williams and Keller 2005). When applied to the lower limb, care must be taken to ensure that the patient is still able to wear shoes during treatment as normal gait pattern is encouraged to maintain an effective calf and foot muscle pump (Williams and Keller 2005).
Bandaging a lymphoedema arm: (approx. 10 mins)
YOUTUBE VIDEO 1
Bandaging a lymphoedema leg: (approx. 10 mins)
www.youtube.com/watch
YOUTUBE VIDEO 2
Inelastic and elastic bandages have been discussed, however more recently, a new bandaging system known as Coban 2 has been developed which can be used an alternative to MLLB. Coban 2 consists of a comfort layer and a compression layer that cohesively bond together (Lamprou et al. 2011). This eliminates the need for a thick padding layer that MLLB requires, resulting in a much less bulky appearance allowing patient’s more mobility and freedom.
Lamprou et al. (2011) conducted a prospective randomised controlled trial comparing Coban 2 with traditional bandaging methods in the treatment of lower limb lymphoedema. The results of this study found Coban 2 to be equally as effective in reducing limb volume.
Franks et al. (2012) studied the use of Coban 2 in arm and leg lymphoedema. Again this study supported the use of Coban 2 in the effective management of lymphoedema with the lower limb showing a greater reduction in swelling.
Although both of the above studies had relatively small sample sizes (40 and 24 participants respectively) resulting in low statistical power, they both showed encouraging results for the use of the new bandaging system.
A multicentre randomised controlled trial with 82 participants (Moffatt et al. 2011) investigating the frequency of application of Coban 2. Results found constant therapeutic effect was maintained when bandages were reapplied every four days. Compared with MLLB, which requires reapplication daily at certain stages of treatment, the Coban 2 bandages allow patients to have more freedom and independence.
Coban 2 bandaging: (approx. 15 mins)
YOUTUBE VIDEO 3
<u<uAs discussed, a number of trials have evaluated the effects of compression bandaging for lymphoedema, which have found positive results. The development of Coban 2 bandages is encouraging, however this still requires larger scale trials to fully evaluate the efficacy.
Now that compression bandaging has been considered, this wiki will now move on to discuss the use of compression garments which are used for the long-term management of limb shape and swelling (Lymphoedema Framework 2006).
Compression Garments/Hosiery
These made-to-measure, handmade garments will be considered once regular limb shape has been restored and the patient’s skin is fully intact and robust enough to tolerate the use of garments (Doherty et al. 2009; Linnitt and Davies 2007).
Although garments are important in the management of lymphoedema, patient and clinician must come to an informed decision regarding the appropriateness of this treatment modality. Doherty et al. (2009) explains that patient’s stage and severity of lymphoedema, shape and size of limb, skin resilience, shape distortion, ability to tolerate, lifestyle, mobility, age, psychological status and dexterity should be assessed and considered prior to measurement and fitting.
Once the progression from bandages to garments has been made, swelling and other symptoms must be monitored. If swelling is not controlled within the first three months of wearing compression garments, clinician and patient should consider further intensive therapy using MLLB (Moffatt et al 2005).
Measurement for hosiery will be undertaken by a qualified health professional, which may be a physiotherapist
PICTURE 3 - meausuring for compression garments
Garments are either constructed as a flat knit or a round knit. Flat knit knitted as a flat garment then joined at the seams, these are generally thicker and firmer. Round knitted garments are viewed as more aesthetically pleasing, as they are thinner than flat knit and are continuously knitted cylindrically without any seams (Lymphoedema Framework 2006).
Once measurement and construction of the garment is completed advice regarding the care at home will be provided in person and leaflets may also be given to the patient (Lymphoedema Framework 2006). If garments are poorly fitted, the swelling may not be contained and damage can occur to the tissues. This could result in discomfort and reduced tolerance leading to patients being unwilling to use compression hosiery as a long-term management option (Doherty et al. 2009).
As well as limb compression garments, if patients have trunk or breast lymphoedema garments or specialised bras can be provided (Lymphoedema Framework 2006).
Activity
Before progressing to the next section please take 15 minutes to check your knowledge of the following:
- List 3 indications for compression therapy
- Name 3 contraindications to compression therapy
- List the components of MLLB
- Understand (discuss with a partner) how bandaging can be altered to suit the patients needs
- Discuss the differences between MLLB and Coban 2 bandaging
- What are the benefits of using Coban 2 over MLLB?
- Name and explain the 2 kinds of compression hosiery
- What should be considered when assessing for compression hosiery?
Skin Care[edit | edit source]
Lymphoedema cause changes to the skin including thickening, hyperkeratosis, hyperpigmentation and papillomatous or verrucous nodules (Nowicki and Siviour 2013). As a consequence of swelling, large skin folds can appear where infections may develop (Lymphoedema Framework 2006). Furthermore infections may arise if the skin becomes damaged or broken, therefore adequate skin care to maintain the integrity and manage any problems that occur is fundamental in the care of people with lymphoedema (Lymphoedema Framework 2006; Wigg and Lee 2015). At both intensive and maintenance stages, it is important to emphasise the need for a skin care regime to maintain the skin integrity.
The Lymphoedema Framework (2012) outlines the main principles of skin care:
- Wash daily
- Ensure skin folds are clean and dry
- Monitor the skin for changes
- Apply emollients
- Avoid scented products
- Using vegetable-based products in tropical climates rather than petrolatum or mineral oil based products
Washing can remove the protective lipid layer that prevents water loss and protects the skin from infection. Therefore emollients are applied to re-establish this layer thus preventing any further water loss and maintaining the barrier to protect from bacteria and other irritants (Lymphoedema Framework 2012).
Any soap that is abrasive or scented are to be avoided, natural or pH neutral soaps are recommended (Lymphoedema Framework 2006; Nowicki and Sivour 2013). This is because normal soaps contain detergents, are often scented and include preservatives, which can irritate or dry the skin.
During assessment, health professionals must inspect the skin condition using palpation and observation to check for any changes or damage (Nowiki and Siviour 2013). If changes have occurred these must be managed and monitored correctly.
Following assessment and cleansing of the skin emollients are applied to maintain skin hydration. These can either be moisturisers, soaps substitutes or bath oils. Moisturisers come in different forms including cream, lotion and ointments (Nowicki and Siviour 2013). The Lymphoedema Framework (2006) recommends the use of ointments, which contain little or no water; this hydrates the skin better than creams and lotions.
Despite the treatment offered infections may still occur that must be managed by thorough skin hygiene, ensuring skin is dried following washing and an anti-fungal powder or cream applied until the infection disappears (Nowicki and Sivour 2013).
The body’s natural response to sunburn is to increase blood flow to the affected area. For people with lymphoedema this will increase the load on an already impaired lymphatic system and may increase swelling. Therefore it is advised that people with lymphoedema take extra care to avoid sunburn (Nowicki and Siviour 2013).
Despite being an integral part of lymphoedema management, some patients experience barriers that prevent adequate skin care and result in infections. James (2011) studied the perceived barriers to skin care, which included physical limitations, expense, poor understanding, anxiety and motivational issues. This indicates that health professionals play a large role in educating patients about the importance of skin care to facilitate self-management. Health professionals should be aware of these potential barriers and be able to overcome them through education and support to facilitate self-management.
Activity
Take 10 minutes-
Can you…
List the main principles of skin care?
Explain why are emollients important?
True or false – is ointment recommended over lotions?
Take 5 minutes to think of how you would explain the importance of skin care to a patient you are treating? If possible discuss this with a partner.
Exercise[edit | edit source]
MacMillian Cancer Support (2013) state that many individuals experience reduced quality of life following cancer treatment due to secondary complications, which can include lymphoedema. Despite the general well known benefits of exercise including reduced risk of chronic diseases and the positive impact it can have on mental health (NHS Choices 2015), studies report that cancer survivors often fail to return to their pre-diagnosis levels of physical activity (Irwin et al. 2003; Harrison et al. 2009).
Traditionally strenuous exercise was discouraged in patients with lymphoedema based on the belief that it may exacerbate the condition (Cheifetz et al. 2010). However recent studies and systematic reviews contradict this statement.
Schmitz et al. (2009) evaluated the effects of a weightlifting programme on 141 participants presenting with breast cancer related upper limb lymphoedema. Due to the relatively large sample size the results of this study are significant in proving that weight lifting did not produce an increase in limb swelling. Furthermore, the study found that the exercise programme resulted in a decrease in lymphoedema exacerbations and symptoms.
Singh et al. (2015) reviewed the effects of single bouts of exercise and regular training with mixed exercise modes on cancer related lymphoedema. Overall this review concluded that independent of the mode of exercise – resistance or aerobic – there were no detrimental effects on the subject’s lymphoedema. Despite showing little effect on lymphoedema, it is important to note that exercise improved the functioning of subjects through improving their ability to carry out activities of daily living. Singh et al. (2015) also reviewed the use of compression garments during exercise. Unfortunately due to the range of effects that wearing compression can have during exercise, there was no definitive answer to whether compression should be worn or not. The authors suggest that this decision should be made on an individual basis considering factors such as stage, severity, and stability of lymphoedema and patient preference.
Further smaller studies found that combined exercise programmes including aerobic and resistance exercises were safe and had no harmful effects for patients with upper limb lymphoedema (Hayes et al. 2009; McKenzie and Kalda 2003). Again both of these studies supported the statement that exercise does not exacerbate lymphoedema but it does have a positive psychological impact, particularly in relation to quality of life.
Kwan et al. (2011) suggest that exercise supervised by a qualified professional i.e. a physiotherapist in the first instance to ensure correct technique and reduce injury risk. Paramanandam and Roberts (2014) made an important point that giving patients a choice in their exercise programme improved their adherence.
Overall it can be concluded from the body of evidence including systematic reviews and large randomised controlled trials that strenuous training as previously thought does not worsen or cause lymphoedema in breast cancer survivors. The benefits of exercise on individual’s strength, functioning and quality of life outweigh any risks. Furthermore, there is little evidence to recommend that one mode of exercise is superior to others, which allows individuals the freedom of choice of exercises. This choice is likely to positively impact the adherence to exercise programmes. Hence physiotherapists and other health professionals should be encouraging their patients with lymphoedema to undertake exercise programmes and make individuals aware of the benefits that exercise can have on physical and mental wellbeing.
Activity
Take 10 minutes to think about the following case study. This can be discussed in groups if you would like to.
Case Study
Lady with (R) UL lymphoedema has completed cancer treatment. She independently manages her lymphoedema with skin care; self massage and wears a compression garment. This lady tells you she would like to get back to her previous levels of activity but is concerned that exercise may make her lymphoedema worse. She asks you for advice about what exercises are safe for her to do.
What advice would you give her?
Can you think of 5 exercises that she could do either at home or in the gym?
Manual and Simple Lymphatic Drainage[edit | edit source]
Emil Vodder came up with the method of manual lymphatic drainage (MLD) in 1936. He stated “MLD along with breathing and relaxation exercises and improved diet would play a key role in lymphatic disorders” (Vodder 1965, pp *** in Williams 2010). This method of massage uses gentle strokes to enhance lymph drainage through lymphatic pathways (Lymphoedema Framework 2006). The treatment is conducted by trained professionals and is used in combination with the other components of decongestive therapy, as it is not enough to be used alone (Lymphoedema Framework 2006).
Indications:
- Swelling at root of the limb
- Trunk or midline swelling – may be used alone in this instance as it could be the only suitable treatment
- Provision of comfort or pain relief
- Adjunct to pain management
(Lymphoedema Framework 2006)
Contraindications:
- Acute cellulitis/erysipelas
- Renal failure
- Unstable hypertension
- Severe cardiac insufficiency
- Hepatic cirrhosis with abdominal fluid
- Superior vena cava obstruction
- Untreated tuberculosis or malaria
- Local contraindications (not to be used at these sites):
- Untreated thyroid dysfunction
- Primary tumours
- Metastases
(Lymphoedema Framework 2006)
Principles and technique:
- Slow repetitive movements
- Aims to increase lymph drainage without altering capillary function
- Alter interstitial pressures by varying hand movements
- Moves proximally to distally
- Incorporates breathing techniques (deep diaphragmatic breathing) to encourage drainage from deep abdominal lymph nodes and vessels
- Up to one hour daily
(Lymphoedema Framework 2006; Williams 2010)
As well as MLD another form of this treatment is Simple Lymphatic Drainage (SLD) - a simplified version of MLD that can be taught to people with lymphoedema or their carers to form part of a self-management programme. MLD can be performed for up to one hour daily, however SLD is performed for 10-20 minutes (Pyke 2010).
Prior to teaching SLD to individuals, health professionals must consider:
- Motivation of the individual
- Dexterity
- Time allocated for teaching which should be progressive
- Written instructions provided
- Check technique competency and how the individual is coping regularly
MLD has been found to reduce limb volume, improve quality of life and lymphoedema symptoms in people with cancer related lymphoedema (Williams et al. 2002). However, this study had a number of flaws limiting its quality, indicating the need for further research in this area. When comparing MLD to SLD, Sitzia et al. (2002) suggests MLD to be more beneficial at reducing limb swelling. However these results were from a small pilot study and did not reach statistical significance.
A more recent systematic review (Huang et al. 2013) evaluated the effects of MLD in preventing and treating breast cancer related lymphoedema. Overall the findings from this review were unable to support the use of MLD in the prevention or treatment of lymphoedema in this patient group.
Although the evidence discussed was unable to support the use of MLD and SLD to treat lymphoedema, the Lymphoedema Framework (2006) still recommend it as one of the cornerstones of decongestive lymphatic therapy. The framework suggests that despite the efficacy not being proven, MLD and SLD both have clear psychological and symptomatic benefits. Pyke (2010) discussed that massage can reduce fear and reassure patients that although their skin is painful and may be damaged it can still be touched.
Overall further evidence is required to fully evaluate the benefits of MLD or SLD in the management of lymphoedema. The benefits may not reach statistical significance but the impact that MLD or SLD could have on an individual’s quality of life are important to consider.
Furthermore SLD, although little evidence to support its use allows the patient some independence, as they are able to take some responsibility for the management of their condition.
Activity
Take 5 minutes -
Questions:
What is the difference between MLD and SLD?
True or false – MLD is started distally and works proximally?
What are the contraindications for MLD and SLD?
Conclusion[edit | edit source]
This section has discussed the management options for people with lymphoedema, focussing on the 4 main cornerstones of decongestive lymphatic therapy.
The evidence base has been discussed along with the recommendations provided by the best practice guidelines of (Lymphoedema Framework 2006).
It is important to note that the treatments discussed are not stand alone therapies. Ideally, the treatment of lymphoedema should contain all components of DLT to manage symptoms and reduce complications (Lymphoedema Framework 2012).
Despite relatively sound evidence base supporting the use of DLT as a whole, when looking into the separate components the quality of trials is not as reliable. As discussed there is a need for larger scale trials to fully evaluate the use of specified treatments in the management of lymphoedema.
Before progressing onto the next section of this wiki, please ensure you have taken the time to answer all the quizzes at the end of each sub-section.
By now you should be able to –
Summarise and explain the physiotherapy management options for people with lymphoedema.
Lymphoedema in Palliative Care[edit | edit source]
Definition and History
[edit | edit source]
When you hear the term palliative care, what does it mean to you? Take a moment to think about this and take note of your answer.
Definition:
The WHO defines palliative care as “an approach that improves the quality of life of patients and their families facing the problem associated with life-threatening illness, through the prevention and relief of suffering by means of early identification and impeccable assessment and treatment of pain and other problems, physical, psychosocial and spiritual” (WHO 2015).
A more general description may define palliative care as any care that helps to alleviate symptoms whether or not there is hope for a cure.
History:
Hospices in the UK and Ireland have been around since the 1900s, however, they were few in number and run by religious foundations to provide care to the poor.
During the 1950s, there was considerable professional and public interest in cancer but the main focus was on curative treatment. Patients who were considered terminal and were dying from cancer were overlooked and were told to go home as there was nothing that could be done or were scattered in various hospital wards, abandoned by doctors.
In the postwar years, a shift began to emerge in the published work with new studies showing both the clinical and social aspects of care for patients who were dying from cancer. This work in oncology helped to shape the worldwide development of palliative care (Clark 2007).
Cicely Saunders may be recognized as the founder of the palliative care movement around the 1970’s.
Having trained as a nurse and then a social worker, in 1959, Saunders qualified in medicine and began working in St. Thomas’s Hospital in London (Baines 2011).
In 1960, Saunders focused her attention on patients who were in the final stages of cancer, especially those patients with complex problems from the point of view of pain and general distress. Her research and writings were built on the individual experiences of her patients and by 1967 she had collected data for 1100 cases, where she described the physical and mental suffering of each patient (Clark 2007). Saunders inspired the concept of total pain which includes physical, emotional, social, mental and spiritual components and believed in constant pain management to relieve suffering.
In 1967, Cicely Saunders opened St. Christopher’s Hospice in London which was the world’s first modern hospice. Here, she brought together large numbers of patients with terminal illness and staff to take care of them (Baines 2011). The hospice quickly became an inspiration and established itself as a centre of excellence, giving equal importance to clinical care, education and research. Research was done on pain control and the administration of strong opiates. These clinical and organizational studies would go on to play a major role in the advancement on palliative care (Clark 2007).
The success of St. Christopher’s Hospice soon became a catalyst for the development of hospices in the UK. During the 1980s, about 10 new hospices were opened a year, some were funded by the NHS and others were privately or charity funded.
There was also a development among hospitals and in 1976 a terminal care team was established in St. Thomas’s Hospital, London. Between 1982 and 1996 the number of hospitals with a multidisciplinary palliative care team or a specialist nurse more than quadrupled to 275 from 5.
During this time, two well known UK charities also assisted in influencing change. The first of these charities was the Macmillan organization which was founded in 1911. During the 1970s the organisation went through a period of phenomenal expansion. The organisation became more involved in palliative care and supporting specialist professional posts, academic positions and service development.
The second charity is the Marie Curie Memorial Foundation, established in 1948. This foundation was involved in creating a domiciliary nursing service for patients with cancer. It created nursing homes and ran a laboratory-based research programme. During the 1980s when new hospices were opening across the UK, the Marie Curie nursing homes evolved into specialist palliative care centres and the charity supported more research and educational activities in palliative care (Clark 2007).
By the 1990s there were over 1000 specialist Macmillan nurses and 5000 Marie Curie nurses working across the UK in palliative care.
By 1987, palliative medicine was established as a subspecialty of general medicine and in 1995 the specialty of palliative care was formally approved (Clark 2007).
Aims of Palliative Care[edit | edit source]
- To maintain quality of life until death
- Provide relief from pain or other symptoms which may cause distress
- Help patients to live as actively as possible
- Help family members to cope during a patient’s illness and during their own bereavement
- Integrate spiritual and psychological aspects into a patient’s care
- Be applied early and in conjunction with other therapies (NHS 2004)
Causes of death[edit | edit source]
When looking at who needs palliative care and who should provide it, we need to look at epidemiology and the causes of death across the world. Epidemiology is important when planning health services as it can provide information about disease and symptom occurrence to guide healthcare needs. Life expectancies vary worldwide. Variations are associated with certain demographic characteristics such as political, occupational, cultural and lifestyle risks while also including gender, ethnicity and genetics. Low-income countries may have lower life expectancy then developed countries. In 2012, life expectancy was estimated globally at 66yrs for men and 71yrs for women. However, in more developed countries, life expectancy is estimated at 76.9years for both sexes and 58.4years in the least developed countries.
graph of the leading causes of death, worldwide for 2011.
This is only a broad reflection of the figures as reported causes vary among countries at different levels of economic development.
Future projections – mortality projections may aid the planning of health services and the knowledge and skills clinicians will require in order to meet the needs of future populations
Service delivery[edit | edit source]
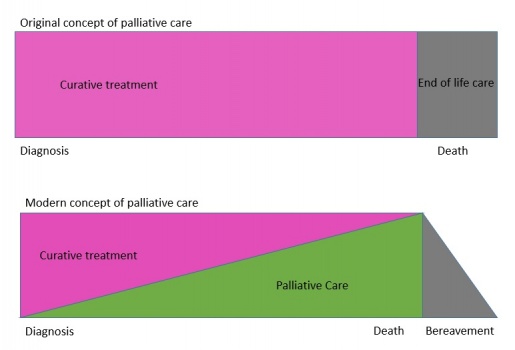
Since the inception of the modern hospice and palliative care movement led by Cicely Saunders, palliative care has continued to evolve, has developed into a medical specialty and is now integrated into mainstream medicine in many countries. Palliative care was originally seen as something only concerned with the end of life but has now been incorporated with other services offered throughout the entire disease trajectory.
Palliative care is now a more seamless process through the disease trajectory, either from the point of diagnosis or at any point in the disease compared to the original concept where care was offered once curative treatment had failed and patients were seen at an end of life stage.
Due to the aging population in developed countries, the burden of disease on healthcare will continue to increase, challenging the knowledge and skills of professionals. With the advances in treatments for cancer and other conditions, patients are living with more co-morbidities and palliative care services must be prepared to provide care over longer periods of time throughout the disease trajectory.
Where care is provided[edit | edit source]
Palliative care takes place in many different settings.
Can you think of any settings where palliative may take place?
Hospices possibly being the most known setting. Hospices can be a ward or unit within a hospital or can be a stand-alone service. Hospices in the UK are generally stand-alone services with the aim of alleviating disease or therapy-related discomfort and stabilising the status of patient by offering psychological and social support. Cicely Saunders once said that hospices should be a welcoming environment and have a sense cheerfulness and peace.
Other settings include hospital palliative support teams, home care teams, palliative care outpatient services and day care centres.
Hospital palliative support teams – provide advice to patients, their family, carers and other clinical staff. The teams provide education and liaise with other services both in and out of hospital. An aim of the team is to alleviate multiple symptoms patients may have by sometime prescribing directly or will advise on the management of symptoms.
Home care teams – provide specialist direct care to patients in their home where they support families and carers. The team may provide specialist advice to GPs, nurses or other clinicians involved in the patient’s case. There is good evidence to show the benefits of home specialist palliative care compared with usual care.
Outpatient services – this service is available for patients who live at home but are able to attend clinics. These services may be offered from a hospital or inpatient palliative care unit. This service can help to introduce patients to the palliative care process earlier where advanced care planning can be put in place. There is little to no evidence on the effect of these services.
Day care centres – these are spaces in hospitals, hospices or the community which are specially designed to provide additional support to patients and their families. Usually patients who attend a day centre are already in the care of a home palliative care team. The nature of the service offered may vary depending on the patient’s needs, from medical/health orientated to more recreational or social services where complementary therapies may also be offered
Who[edit | edit source]
Palliative care uses a team approach to affirm life and help support patients to live as actively as possible until death by enhancing their quality of life. Care should be offered as patient needs develop and should be an integral part of care in any setting (WHO 2004). Care is focused on controlling pain and other symptoms based not on disease prognosis but on the needs of the patient and must be flexible to adapt to these needs (WHO 2004).
Professionals involved – multidisciplinary teams are the key to all palliative care services. Specialist palliative nurses or physicians are usually part of all services but other professionals may be involved depending on the service. Social workers, physiotherapists, occupational therapists, psychologists, pharmacists and religious officials may be part of a patient’s care.
Physiotherapy in pallaitive care[edit | edit source]
Physiotherapists help people to maximise their potential and maintain or improve their quality of life. In palliative care, physiotherapists have many roles.
These include:
- Assessment
- Symptom management
- Education and communication
- Rehabilitation and function
- Some psychological aspects of care
Assessment
Palliative and specialised palliative care services offer a multidisciplinary team (MDT) approach. Key principles must be applied to the assessment such as considering the patient as a whole person, focusing on quality of life rather than quantity, management should be decided by the patient and there should be good communication for effective assessment and management (ILF 2010). The assessment should involve a patient-centred approach in order to elicit the physical, social and psychological needs of the patient (Todd 2009b).
The assessment should aim to:
- Understand the patient’s main concerns, goals and priorities
- Help the clinician understand the main cause of and the mechanisms behind the swelling
- Understand the underlying condition and how quickly it is progressing.
Oedema is a direct result of multiple factors relating to a terminal illness. It can be distressing for patients and a management challenge for health professionals. It is estimated that 5-10% of new referrals to palliative care have oedema but this is also thought to be underestimated (ILF 2010). Heavy and swollen limbs can cause proximal pain while patients with active malignancy may experience neuropathic pain due to nerve compression. Up to 67% of patients experience pain as a result of oedema. For patients, lymphoedema may be seen as a constant reminder of their cancer or illness. The swollen limb is heavy and uncomfortable which leads to a reduction in mobility and function (Todd 2009a).
The assessment includes a full history of the oedema including the following areas:
Physical
- A new complication, how long it has been present, factors that make it worse or better
- Symptoms causing the swelling, affect it is having on physical abilities, any other symptoms such as pain
Social
- How physical abilities have affected patient’s role
- The consequences – financial, self-care, participation
- What support the patient has from family or friends
Psychological
- Responses to deteriorating health – feelings of worthlessness
- Sadness or anxiety, denial as a coping strategy
- What the patient’s coping strategies are and a history of mental health
Spiritual
- Looking into the patient’s range of thoughts, questions or concerns and helping them through any existential questions
All areas are combined with a history of the illness to understand the underlying causes better and a history of medication (ILF 2010).
An examination is then carried out and baseline measurements are taken. This helps to plan the programme of care, assess the response to treatment, identifies risks of complications and may show signs that confirm the cause of the oedema. However, the assessment is ongoing with constant reviewing as the disease progresses and patient priorities change (Todd 2009a). From the assessment, the priority will be to negotiate a care plan based on the patient’s problems and the best approach to alleviate these problems (Todd 2009b).
Patient goals and management [edit | edit source]
Patient’s goals may often be unrealistic and interventions can sometimes have little or no effect on swelling (Todd 2009b). Management plans are tailor made for the patient, taking into consideration their priorities, the specific aggravating factors or mechanisms of the disease and the anticipated progression of the oedema and condition (ILF 2010).
Due to the unpredictability of disease progression, patient goals may focus on the short term instead of having concern for future complications. Some realistic goals may be improving discomfort by limiting increasing oedema, reducing pain or reducing lymphorrhea (ILF 2010). When considering treatments, perseverance and adaptability are a necessity, any small improvements in swelling can be significant for a patient and make a difference to their quality of life in the later stages (ILF 2010).
Traditionally Lymphoedema is managed with complete decongestive therapy (CDT) and is based on four pillars of care: compression, massage, skin care and exercise (Todd 2009b). However, routine intensive management using decongestive lymphatic therapy may not be appropriate due to weakness and frailty so must be modified and adapted. A palliative approach is adopted in order to relieve symptoms, improve quality of life and reduce any risks associated with the oedema. The benefit of the treatment must outweigh the potential burden and take the patient’s needs into consideration (ILF 2010).
Challenges in Physiotherapy[edit | edit source]
Treatment[edit | edit source]
The main aim of palliative rehabilitation is to set treatment goals that allow a patient to maintain or improve functions and delay the effects of their disease for as long as possible. To lose their functional ability can cause a patient to view themselves differently and lose their independence which can be very detrimental to a patient. Therefore, physiotherapists have a huge role in helping patients to maintain their independence and achieve other goals they may have (Frymark et al. 2009).
Working as a physiotherapist within a palliative care setting can be very challenging as you must have a solid knowledge base about the disease your patient has as well as common physiotherapeutic treatment methods which you will have to adapt for a palliative patient as the treatment goal will be different. Goals need to be short-term and adaptable due to the changing nature of a palliative condition. Therefore, sometimes it is necessary to do a new assessment every time you see a patient due to how much their condition can vary (Frymark et al. 2009).
When it comes to specifically lymphoedema patients, physiotherapists usually work towards the goal of restoring ‘near normal’ limb shape and size. However, in a palliative setting this goal is often unrealistic, therefore, goal setting must be sensible and based on what the patient needs or wants. When goal setting the patient should; have close involvement with the physiotherapist and other health professionals involved in their care and be educated on how to self-manage symptoms. Acceptable palliative goals may include, slowing down the progression of swelling and reducing other symptoms associated with lymphoedema (ILF 2010).
Patients may have dramatically reduced physical capacity. If their illness has compromised neurologic structures, they may be plegic or paretic, and therefore unable to self- bandage or perform remedial exercises. Further, they may be significantly limited by symptoms such as fatigue and dyspnoea due to marked deconditioning. The coordination, dexterity and strength requirements for bandaging and donning compression garments can elude even healthy patients; therefore, these potential difficulties should be considered when formulating a management plan for terminally ill patients (ILF 2010).
Management[edit | edit source]
Recent Related Research (from Pubmed)[edit | edit source]
Extension:RSS -- Error: Not a valid URL: Feed goes here!!|charset=UTF-8|short|max=10
References[edit | edit source]
References will automatically be added here, see adding references tutorial. .</div>