Hemochromatosis
Original Editors - Jill Heil & Jillian Redlinger from Bellarmine University's Pathophysiology of Complex Patient Problems project.
Top Contributors - Jill Heil, Jillian Redlinger, Kim Jackson, Lucinda hampton, Admin, Elaine Lonnemann, Anna Fuhrmann, 127.0.0.1, Wendy Walker and WikiSysop
Introduction[edit | edit source]
Hemochromatosis is a condition that causes excess iron deposition and can lead to multiple organ dysfunction.[1]. Individuals with hemochromatosis lack an effective way to remove excess iron, and the iron begins to accumulate with subsequent development of fibrosis in the liver, pancreas, skin, heart, and other organs[2]. Excess iron accumulation in the body promotes oxidation and causes tissue injury, fatigue, arthralgia or arthritis, and skin changes. Complications can include hepatomegaly, diabetes, impotence (males), pulmonary involvement, and cardiac myopathy[2].
Epidemiology[edit | edit source]
- The most common autosomal recessive disorder in whites, being present in 1 in 300 to 500 individuals, with the white population having a six times higher risk of developing the disease than blacks.
- Hereditary hemochromatosis type 2, 3, and 4 occur globally however type 1 is mainly found in people of northern European descent.[3]
- Occurs 5-10 times more often in men than women; this is due to women losing blood through menstruation and pregnanacy[2].
- Symptoms occur in men >50 years and are rarely symptomatic before 30 years of age[2] whilst women experience symptoms around age 60[2].
Characteristics/Clinical Presentation[edit | edit source]
Historically, hemochromatosis was identified by a classic clinical triad which includes an enlarged liver or hepatomegaly, skin hyperpigmentation, and diabetes. However, hemochromatosis has different signs and symptoms, affecting many systems. Signs include:[2].
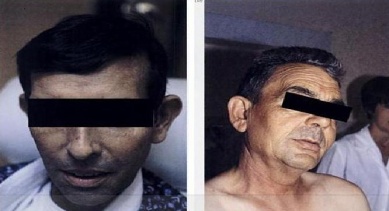
- Hyperpigmented skin "bronze": 90%
- Hepatomegaly: 90%
- Arthralgia: 50%
- Diabetes: 30%
- Heart failure/arrhythmia: 15%
- Hypogonadotropic hypogonadism[4]
Early symptoms promoting a visit to the physician include severe fatigue (74%), impotence (45%), and arthralgia (44%)[5]. Often times, when symptoms begin to appear, it is too late because iron accumulation has caused irreversible tissue or end-organ damage in the heart, liver, endocrine glands, skin, joints, bone, and pancreas. Hemochromatosis is the most common genetic disorder causing liver failure[2].
Diagnostic Tests/Lab Tests/Lab Values[edit | edit source]
It is recommended by the American Association for the Study of Liver Diseases (AASLD) that high risk groups should be screened for hemochromatosis. High risk individuals include those having a history suggestive of organ involvement, a familial history or hereditary hemochromatosis, and an individual with biochemical or radiologic abnormalities suggesting the possibility of iron overload. The Center for Disease Control and Prevention (CDC) suggests an individual be screened for hemochromatosis when their history suggests iron overload, they have a family history, or are symptomatic[6].
A blood test testing the amount of iron in the blood can suggest a diagnosis and the need for further treatment[7] The blood test should be done on individuals with signs and symptoms of iron overload and on first-degree relatives of patients with hereditary hemochromatosis older than 20 years[6]. It should be done after an overnight fast and the measurement of transferring saturation will tell if there is an iron overload[6]. Measuring serum ferritin at the same time will increase the predictive accuracy for diagnosis of iron overload[6].
The most definitive test is a liver biopsy[7]
Organ abnormalities may be revealed from a CT (computerized topography) scan or from an MRI (magnetic resonance imaging), which may help determine the severity of the disorder[7].
Etiology[edit | edit source]
Retained iron is mainly accumulates in the parenchymal cells in hereditary hemochromatosis, and in transfusional hemochromatosis it is mainly is deposited in the reticuloendothelial cells. This surplus iron is deposited in the cells as hemosiderin, eventually causing cell death and replacement of these cells by fibrosis causeing destruction or impairment of organ function.[8]
Systemic Involvement[edit | edit source]
- Arthropathy, the most common presenting symptom in women with hemochromatosis. It mostly occurs with arthralgias of the metacarpophalangeal joints of the hands [9].
- CPPD disease Calcium pyrophosphate dihydrate crystal deposition (CPPD) disease also known as Ppseudogout. There are about 30% of patients with hemochromatosis arthritis who have CPPD crystals recovered from synovial fluid[10].
- Endocrine Problems. During the late stages of hemochromatosis, Impotence and testicular atrophy are a common problem [9]
- Cardiac Disease. Congestive Heart Failure is a relatively uncommon manifestation of hemochromatosis, but it sometimes occurs in the later stages[9].
- Cirrhosis of the liver. Excess iron in the liver may cause liver problems[7].
Medical Management[edit | edit source]
The most important determinant of prognosis in hemochromatosis is the timing of diagnosis. The earlier the disease is detected the earlier treatment can be initiated and a decreased risk of hepatic fibrosis may be expected. A patient with hemochromotosis can expect to live a normal life if diagnosed early and if they maintain adequate iron levels.
- Therapeutic Phlebotomy: In order to reduce the amount of iron in the blood, therapeutic phlebotomy, also known as blood letting can be done until iron saturation is less than 50% and ferritin level becomes less than 50 ng/mL[1]. Initially, one-half liter of blood is removed from the body each week until the body iron level is normal. This may require many months or even years to accomplish. After that, less frequent phlebotomy is needed to maintain normal iron levels (See Figure 2) . How often you need this procedure depends on symptoms, levels of hemoglobin and serum ferritin, and how much iron you take in your diet[11].
Figure 2[12]. Schematic of Phlebotomy Need.
- Chelation Therapy: In patients with hemochromatosis who have heart disease, anemia, or poor venous access, treatment with iron chelation agents is recommended over therapeutic phlebotomy. This medicine inhibits intestinal absorption of iron, chelators of iron, hepcidin, or ferroportin supplementation. Iron chelation agents include Deferasirox and Dendrimers (see medicine section).
- Surgical Intervention: In patients with end stage liver disease or severe arthroplasty, surgery may be necessary. When end-stage liver disease progresses despite iron-reduction therapy, liver transplantation is the only therapeutic option. Another indication for liver transplantation is the development of hepatocellular carcinoma[13].
Physical Therapy Management[edit | edit source]
Mary K. Allen emphasizes the importance of recognizing and understanding early symptoms and management of a patient with hereditary hemochromatosis so therapists will consider the disorder as a differential diagnosis or co-morbidity which will affect the physical therapy treatment received[14].
- Many of the early symptoms which initiate a physician visit are arthralgia and fatigue. These complaints can mimic other conditions such as arthritis or fibromyalgia which can make diagnosis challenging[14].
- The case report included in the article explains how the medical management of hereditary hemochromatosis can limit a patient’s tolerance to exercise after trauma. A physical therapist may need to take on the responsibility to contact the managing hematologist about an injury when a patient initiates of therapy[14].
- When evaluating a patient with hereditary hemochromatosis, a PT should be aware of the insidious onset of fatigue and joint pain. Also note that hereditary hemochromatosis may be a differential diagnosis. The exercise tolerance needs to be monitored, and interventions may need to be changed to accommodate if there are symptoms of low serum iron levels, such as fatigue and shortness of breath. Cardiac changes may also present[14].
Hereditary hemochromatosis may cause both systolic and diastolic dysfunction in patients. Once systolic dysfunction is documented, exercise capacity becomes compromised[15].
There has been no data studying the exercise capacity in hereditary hemochromatosis during the asymptomatic stage of this disorder so it is assumed that the exercise capacity and activity available should be no different than that for the general population[15].
The removal of iron through phlebotomy treatment for up to 5 years has shown no affect on exercise capacity with subjects who have hereditary hemochromatosis compared with control subjects[16].
Patients with hereditary hemochromatosis are continually exposed to elevated levels of oxidative stress even as iron levels improve which affect aerobic exercise capacity through modulation of the left ventricular diastolic function[16]. There is further studies which need to be done in this area but it is something to keep in mind when treating patients with hemochromatosis.
Dietary Management[edit | edit source]
Hemochromatosis can be better managed with some lifestyle changes. A nutritionist is a good resource for patients with this disease. Affected individuals are instructed to avoid ingesting[17]:
- foods with high levels of iron such as red meat or organ meat
- anything iron fortified
- iron supplements and multivitamins containing iron
- Ascorbic acid (Vitamin C) - accelerates the intestinal absorption of inorganic iron
- Alcohol - accelerates the absorption of dietary iron. Alcoholism occurs in 40% of patients with hemochromatosis[2]. Alcohol also, increases the risk of liver disease and so should be avoided completely if you have hemochromatosis and liver disease.
- Raw shellfish - can be contaminated with Vibrio yulnificus and can cause sepsis in patients with hemochromatosis.
Case Reports/ Case Studies[edit | edit source]
- PT and Hemochromatosis[18] www.ncbi.nlm.nih.gov/pmc/articles/PMC2911836/.
- Case study of a 69 y/o women with no family history of hemochromatosis, but signs and symptoms present. http://www.cdc.gov/ncbddd/hemochromatosis/training/case_studies/case_2/index.html[19].
- Case Study of a 36y/o man with no family history of hemochromatosis, but elevted serum ferritin levels found. http://www.cdc.gov/ncbddd/hemochromatosis/training/case_studies/case_5/index.html[19].
- Hemochromatosis and Diabetes: http://clinical.diabetesjournals.org/content/22/2/101.full[20].
Resources[edit | edit source]
References[edit | edit source]
- ↑ 1.0 1.1 Goodman CC, Boissonnault WG, Fuller KS. Pathology: Implications for the Physical Therapist 2nd ed. Philadephia: Suanders, 2003.
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 2.6 2.7 Goodman CC, Snyder TK. Differential Diagnosis for Physical Therapists: Screening for Referral 4th ed. St. Louis: Saunders Elsevier, 2007.
- ↑ Porter JL, Rawla P. Hemochromatosis. 2022 Jun 11. StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing. 2022.Available:https://www.ncbi.nlm.nih.gov/books/NBK430862/ (accessed 28.1.2023)
- ↑ Radiopedia Haemochromatosis Available:https://radiopaedia.org/articles/haemochromatosis (accessed 28.1.2023)
- ↑ Duchini A, Katz J. Medscape Reference: History and Physical Examination. http://emedicine.medscape.com/article/177216-clinical (accessed 29 March 2012).
- ↑ 6.0 6.1 6.2 6.3 Hemochromatosis Clinical Presentation. Presentation. http://emedicine.medscape.com/article/177216-clinical#a0256 (accessed 29 March 2012).
- ↑ 7.0 7.1 7.2 7.3 Margolis, S. The Johns Hopkins Complete Guide to Symptoms and Remedies. New York: Tess Press, 2004.
- ↑ Porter JL, Rawla P. Hemochromatosis. 2022 Jun 11. StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing. 2022.Available:https://www.ncbi.nlm.nih.gov/books/NBK430862/ (accessed 29.1.2023)
- ↑ 9.0 9.1 9.2 Hemochromatosis: Clinical Features of Hemochromatosis. http://www.medscape.com/viewarticle/421519_3 (accessed 29 March 2012).
- ↑ Stevens SM, Edwards CQ. Identifying and managing hemochromatosis Arthropathy: Nonspecific symptoms and overlapping conditions complicate the diagnosis. http://www.musculoskeletalnetwork.com/display/article/1145622/1391837 (accessed 29 March 2012).
- ↑ PubMed Health, U.S. National library of Medicine http://www.ncbi.nlm.nih.gov/pubmedhealth/PMH0001368/ (accessed 29 March 2012)
- ↑ ChelationtherapyOnline. Iron overload and Hemochromatosis Information for Patients and Their Families. http://www.chelationtherapyonline.com/anatomy/p6.htm (assessed 29 March 2012).
- ↑ Duchini A, Katz J. Medscape Reference: Treatment. http://emedicine.medscape.com/article/177216-treatment (accessed 29 March 2012)
- ↑ 14.0 14.1 14.2 14.3 Allen, MK. Hereditary Hemochromatosis: A Literature Review and Case Report. Physiotherapy Canada 2010; 62:276-284.
- ↑ 15.0 15.1 Shizukuda Y, Bolan CD, Tripodi DJ, Yau Y, Smith KP, Arena R, Waclawiw MA, Leitman SF, Rosing DR. Exercise Capacity of Cardiac Asymptomatic Hereditary Hemochromatosis Subjects. Official Journal of the American College of Sports Medicine 2007; 39:3-7
- ↑ 16.0 16.1 Shizukuda Y, Smith KP, Tripodi DJ, Arena R, Yau Y, Bolan CD, Waclawiw MA, Leitman SF, Rosing DR. Changes in Exercise Capacity in Subjects with Cardiac Asymptomatic Hereditary Hemochromatosis During a Follow-Up After 5 yrs. American Journal of Physical Medicine Rehabilitation 2012;9: 1-7.
- ↑ Harms R. Lifestyle and home remedies. http://www.mayoclinic.com/health/hemochromatosis/DS00455/DSECTION=lifestyle-and-home-remedies (accessed 29 March 2012).
- ↑ Allen MK. Hereditary Hemochromatosis: A Literature Review and Case Report. Physiother Can 2010; 62: 276-284. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2911836/ (accessed 29 March 2012).
- ↑ 19.0 19.1 Centers for Disease Control and Prevention. Hemochromatosis (Iron Storage Disease): Training and Education- Case Studies. http://www.cdc.gov/ncbddd/hemochromatosis/training/case_studies/case_2/index.html (accessed 29 March 2012)
- ↑ Capell P. American Diabetes Association: Hemochromatosis in Type 2 Diabetes. http://clinical.diabetesjournals.org/content/22/2/101.full (accessed 29 March 2012)