Chronic Inflammatory Demyelinating Polyneuropathy (CIDP): Difference between revisions
No edit summary |
Kim Jackson (talk | contribs) No edit summary |
||
| (36 intermediate revisions by 7 users not shown) | |||
| Line 1: | Line 1: | ||
<div class="editorbox"> '''Original Editor '''- [[User:Megan Grubb|Megan Grubb]], [[User:Sydney Cudmore|Sydney Cudmore]], [[User:Tania Bhogal|Tania Bhogal]], and [[User:Caitlyn Zavitz|Caitlyn Zavitz]] as part of the [[Queen's University Neuromotor Function Project]]<br> | |||
<div class="editorbox"> | |||
'''Original Editor '''- | |||
'''Top Contributors''' - {{Special:Contributors/{{FULLPAGENAME}}}} | '''Top Contributors''' - {{Special:Contributors/{{FULLPAGENAME}}}} | ||
</div> | </div> | ||
== Introduction == | |||
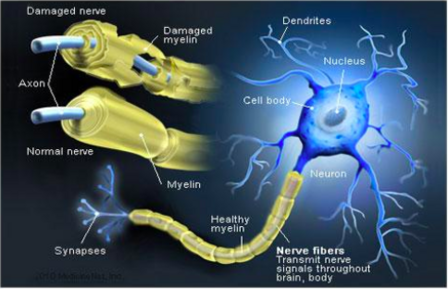
== | [[File:Demyelination of Nerve 1.png|thumb|448x448px|Demyelination of Nerve]] | ||
Chronic inflammatory demyelinating polyneuropathy (CIDP) is | Chronic inflammatory demyelinating polyneuropathy (CIDP) is an acquired [[Demyelinating Disorders|demyelinating disease]] involving peripheral [[Neurone|nerves]], and is generally considered the chronic counterpart to [[Guillain-Barre Syndrome|Guillain-Barré syndrome]] (GBS). About 16% of the patients present with acute GBS. | ||
Patients typically present with a gradual and protracted (>2 months) symmetrical weakness, [[balance]] problems, impaired [[sensation]] and diminished [[reflexes]] and sensory changes. | |||
[[ | |||
* The long term nature of this condition leads to abnormalities in [[gait]] and impairments in both psychological and social functioning. | |||
* Prognosis is variable and depends on age, clinical course, responsiveness to treatment, and electrophysiological findings. <ref name=":11">Radiopedia Chronic inflammatory demyelinating polyneuropathy Available:https://radiopaedia.org/articles/chronic-inflammatory-demyelinating-polyneuropathy (accessed 23.9.2022)</ref><ref name=":0">Janssen J, Bunce M, Nixon J, Dunbar M, Jones S, Benstead J et al. A clinical case series investigating the effectiveness of an exercise intervention in chronic inflammatory demyelinating polyneuropathy. Physiotherapy Practice and Research. 2018;39(1):37-44</ref> <ref name=":1">Latov N. Diagnosis of CIDP. Neurology. 2002;59(6):S2-S6.</ref><ref name=":12">Gogia B, Rai PK. Chronic inflammatory demyelinating polyradiculoneuropathy. Available:https://www.statpearls.com/ArticleLibrary/viewarticle/119994 (accessed 23.9.2022)</ref> | |||
< | This is a helpful 3.5 minute video. | ||
{{#ev:youtube|v=WJVVYk1sg0I|300}}<ref>GBS-CIDP Foundation International | |||
. Chronic Inflammatory Demyelinating Polyneuropathy (CIDP) 101 . Available from:https://www.youtube.com/watch?v=WJVVYk1sg0I [last accessed 23.9.2022]</ref> | |||
== Pathophysiology == | |||
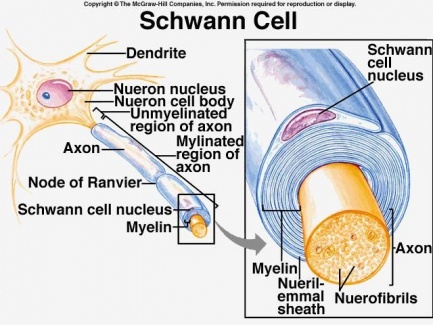
[[File:Schwann Cell 1.jpg|<ref>Purpose of Schwann Cells. (2018). [image] Available at: <nowiki>https://socratic.org/questions/in-human-anatomy-what-is-the-purpose-of-the-schwann-cells</nowiki> [Accessed 7 May 2018].</ref>|alt=|right|frameless|433x433px]] | |||
Chronic inflammatory demyelinating polyneuropathy is an acquired demyelinating disease of the peripheral [[Introduction to Neuroanatomy|nervous system]]. | |||
Surrounding peripheral nerves is a white, fatty layer of myelin that is made up of [[Glial Cells|glial cell]]<nowiki/>s, termed Scwhann cells. The myelin coating generated by the Schwann cells assists the [[axons]] in transmitting faster neural impulses to receptor sites, such as organs and [[Muscle|muscles]]. | |||
[[ | |||
# In CIDP this myelin around the nerves is destroyed resulting in changes of denervation in the supplied muscles. | |||
# Affected nerves demonstrate segmental infiltration with inflammatory cells ([[lymphocytes]]) and demyelination. | |||
# With time there is proliferation of Schwann cells and deposition of [[collagen]], which causes thickening of the nerve and a characteristic onion bulb appearance. <ref name=":11" /><ref>Blumenfeld H. Neuroanatomy through clinical cases. 2nd ed. Sunderland, MA: Sinauer Associates, Inc.; 2010</ref> | |||
== Etiology == | |||
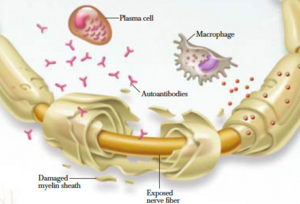
[[File:Damaged Axon.png|thumb|Damaged axon]] | |||
CIDP is an [[Autoimmune Disorders|autoimmune disorder]]. | |||
* It involves both [[T Cells|T cell]]-mediated and humoral immune mechanisms by targeting the myelin of the peripheral nervous system. | |||
* Classical CIDP is idiopathic, but it has variants that can be seen in association with a neoplastic process, [[HIV/AIDS|HIV]] infections, and chronic history of [[Diabetes Mellitus Type 2|diabetes mellitus type II]].<ref name=":12" /> | |||
== Epidemiology == | |||
Overall prevalence has been reported around 0.8 to 8.9 per 100,000 and increases with advancing age (peak incidence of 40 to 60 years of age). CIDP predominantly affects males more than females with a ratio of 2:1 CIDP is suspected to be vastly underestimated, in part due to underreporting, uncertainty in making the diagnosis and diagnostic criteria inconsistencies.<ref name=":12" /> <ref name=":1" /> | |||
== | == Clinical Presentation == | ||
Individuals often report difficulty with walking, climbing stairs, balance and manual dexterity, which is attributed to the progressive, symmetric limb weakness and sensory loss. It is commonly seen to affect proximal muscles in the early stages and then progressively move to the distal limbs. Numbing, buzzing and tingling may be characteristic of sensory loss reported by patients and neuropathic pain is less often reported<ref name=":5" />. | Individuals often report difficulty with [[Walking - Muscles Used|walking]], climbing stairs, balance and manual dexterity, which is attributed to the progressive, symmetric limb weakness and sensory loss. It is commonly seen to affect proximal muscles in the early stages and then progressively move to the distal limbs. Numbing, buzzing and tingling may be characteristic of sensory loss reported by patients and neuropathic pain is less often reported<ref name=":5">Gorson K. An update on the management of chronic inflammatory demyelinating polyneuropathy. Therapeutic Advances in Neurological Disorders. 2012;5(6):359-373</ref>. | ||
On the contrary, some individuals may not present with these symptoms, which makes diagnosis much more difficult and can take many years, leaving patients with no answers and increased frustration<ref name=":6">Wolfe, G. and Greer, M. (2015). Diagnosing and Treating CIDP - GBS/CIDP Foundation International. [online] GBS/CIDP Foundation International. Available at: <nowiki>https://www.gbs-cidp.org/ecomm/diagnosing-and-treating-cidp/</nowiki> [Accessed 7 May 2018].</ref>. | On the contrary, some individuals may not present with these symptoms, which makes diagnosis much more difficult and can take many years, leaving patients with no answers and increased frustration<ref name=":6">Wolfe, G. and Greer, M. (2015). Diagnosing and Treating CIDP - GBS/CIDP Foundation International. [online] GBS/CIDP Foundation International. Available at: <nowiki>https://www.gbs-cidp.org/ecomm/diagnosing-and-treating-cidp/</nowiki> [Accessed 7 May 2018].</ref>. | ||
| Line 53: | Line 48: | ||
# Nerve conduction evidence of a primary demyelinating neuropathy | # Nerve conduction evidence of a primary demyelinating neuropathy | ||
== | == Differential Diagnosis == | ||
Differential diagnoses include:<ref name=":2">Lewis R. Chronic Inflammatory Demyelinating Polyneuropathy. Neurol Clin. 2007;25(1):71-87. doi:10.1016/j.ncl.2006.11.003.</ref><ref name=":4">Koller H, Kieseier B, Jander S, Hartung H. Chronic Inflammatory Demyelinating Polyneuropathy. The New England Journal of Medicine. 2005;352:1343-1356</ref> | |||
* [[Guillain-Barre Syndrome|Guillain-Barre syndrome (GBS)]]: acute presentation of CIDP can be similar; difficult to differentiate in the first 6 weeks; after 6-8 weeks GBS should be improving whereas CIDP will demonstrate chronic inflammation. <ref name=":11" /> | |||
* [[Multifocal Motor Neuropathy|Multifocal motor neuropathy]] | |||
*[[Diabetic Neuropathy|Diabetic polyneuropathy]] | |||
*[[Fibromyalgia]] | |||
*[[Multiple Sclerosis (MS)|Multiple sclerosis]] | |||
*[[Amyotrophic Lateral Sclerosis |Amyotrophic lateral sclerosis (ALS)]] | |||
* [[Neuropathies|Neuropathy]] associated with: nutritional deficiencies, critical illness, toxins, systemic inflammatory diseases. | |||
== Diagnosis == | |||
The diagnosis and management of CIDP is complex and requires an interprofessional inpatient and outpatient team that includes a general neurologist, neuromuscular specialist, intensivist, physical and rehabilitation specialist, pain management specialist, physical therapist, occupational therapist, psychiatrist, social workers, and case management staff <ref name=":12" /> | |||
It is not uncommon for CIDP to go undiagnosed for months to years depending on a patient's symptoms. This could be due to symptoms not being prominent enough to affect an individual's day to day routine and cause concern to visit their physician. In addition, the presence of non debilitating symptoms makes it difficult to give a definitive diagnosis of CIDP in the early stages, even after follow up with their physician<ref name=":6" />. | |||
== Management/Interventions == | |||
Treatment options for CIDP include [[Corticosteroid Medication|corticosteroid]]<nowiki/>s, intravenous [[Immunoglobulins (Ig)|immunoglobulin]]<nowiki/>s (IVIG), and plasma exchange (PLEX). Corticosteroids are used as bridge therapy due to long term side effects. Serial IVIG and plasmapheresis are the mainstays of therapy. Patients who respond to treatment must continue until their condition is stabilized or maximum improvement occurs. Improvements are measured with comparable signs such as improvement in sensation, strength and the performance of activities of daily living ([[Activities of Daily Living|ADLs]]). Further treatment/maintenance therapy is provided to assist the individual with the intention to reduce the frequency of relapses and slow disease progression. Treatments also aim to reduce patient symptoms, such as weakness and pain, and improve overall functional status. <ref name=":12" /><ref name=":4" /><ref name=":5" />. | |||
Occupational or physiotherapists can prescribe gait aids, specialized tools and ankle-foot [[Introduction to Orthotics]], if necessary, to assist individuals with functional tools for ADL’s and ambulation. | |||
Psychologists may also be an important part of the interprofessional team as they can address symptoms that often accompany a diagnosis of a [[Chronic Disease|chronic disease]] and change in functional status, such as [[depression]], anxiety or frustration<ref name=":5" />. | |||
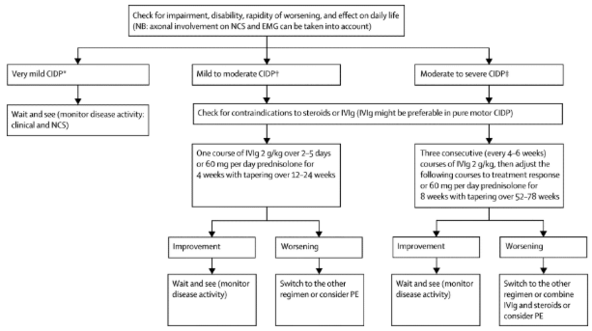
The following describes the process of choosing medical management as suggested by Vallat<ref name=":8">Vallat J-M, Sommer C, Magy L. Chronic inflammatory demyelinating polyradiculoneuropathy: diagnostic and therapeutic challenges for a treatable condition. The Lancet Neurology. 2010;9(4):402–12</ref>: | The following describes the process of choosing medical management as suggested by Vallat<ref name=":8">Vallat J-M, Sommer C, Magy L. Chronic inflammatory demyelinating polyradiculoneuropathy: diagnostic and therapeutic challenges for a treatable condition. The Lancet Neurology. 2010;9(4):402–12</ref>: | ||
[[File:Figure 2..png|center | [[File:Figure 2..png|center|<ref>Vallat J-M, Sommer C, Magy L. Chronic inflammatory demyelinating polyradiculoneuropathy: diagnostic and therapeutic challenges for a treatable condition. The Lancet Neurology. 2010;9(4):402–12</ref>|alt=|frameless|592x592px]] | ||
== | == Physiotherapy Management == | ||
[[File:Manual Therapy to knee joint.jpg|thumb|<ref>Manual Therapy – Foul Bay Physio [Internet]. Foulbayphysio.com. 2018 [cited 7 May 2018]. Available from: <nowiki>http://www.foulbayphysio.com/service/manual-therapy/</nowiki></ref>]] | [[File:Manual Therapy to knee joint.jpg|thumb|<ref>Manual Therapy – Foul Bay Physio [Internet]. Foulbayphysio.com. 2018 [cited 7 May 2018]. Available from: <nowiki>http://www.foulbayphysio.com/service/manual-therapy/</nowiki></ref>]] | ||
A physiotherapist's role in the treatment of CIDP needs to be very specialized to each individual. Physiotherapists prescribe gait aids that may assist with balance and ambulation during ADL’s. Manual therapy may also be provided when indicated to prevent joint contractures and maintain available ROM. Since many individuals with CIDP have difficulty with balance and walking, gait retraining and exercise programs prove to be beneficial at increasing efficiency and endurance<ref name=":5" />. | A physiotherapist's role in the treatment of CIDP needs to be very specialized to each individual. Physiotherapists prescribe gait aids that may assist with balance and ambulation during ADL’s. [[Manual Therapy|Manual therapy]] may also be provided when indicated to prevent joint contractures and maintain available ROM. Since many individuals with CIDP have difficulty with balance and walking, [[Gait Disturbances|gait]] retraining and [[Therapeutic Exercise|exercise]] programs prove to be beneficial at increasing efficiency and endurance<ref name=":5" />. | ||
Exercises that promote muscle strengthening and aerobic conditioning are important once patients have received clearance from a physician for an exercise program<ref name=":9">Hansen M. Exercise for CIDP. 2010</ref>. Exercise parameters for CIDP patients also need to be individually tailored as it is important that individuals don’t over exert themselves. As a result of the demyelination of axons the body is unable to recruit as many muscle fibres to complete a task, which may cause the muscle fibres that are engaged to be overworked. Some delayed onset muscle soreness is expected to occur, but pain that persists longer than 12-48 hours, with or without a loss of strength, is an indication that the F.I.T.T. parameters need to be adjusted because the patient was overexerted<ref name=":9" />. It also crucial to assess that CIDP patients can complete | Exercises that promote muscle [[Strength Training|strengthening]] and [[Aerobic Exercise|aerobic]] conditioning are important once patients have received clearance from a physician for an exercise program<ref name=":9">Hansen M. Exercise for CIDP. 2010</ref>. Exercise parameters for CIDP patients also need to be individually tailored as it is important that individuals don’t over-exert themselves. As a result of the demyelination of axons the body is unable to recruit as many muscle fibres to complete a task, which may cause the muscle fibres that are engaged to be overworked. Some delayed onset muscle soreness is expected to occur, but pain that persists longer than 12-48 hours, with or without a loss of strength, is an indication that the [[FITT Principle|F.I.T.T.]] parameters need to be adjusted because the patient was overexerted<ref name=":9" />. It also crucial to assess that CIDP patients can complete motion against gravity before applying external resistance to the movement, as it is important to have baseline muscle control before progressing exercises<ref name=":9" />. | ||
Recent studies on the effects of exercise programs for patients with CIDP have provided good insight and direction for future physiotherapy treatments. Janssen et al, (2018)<ref name=":0" /> completed an intervention that was designed around the [https://www. | Recent studies on the effects of exercise programs for patients with CIDP have provided good insight and direction for future physiotherapy treatments. Janssen et al, (2018)<ref name=":0" /> completed an intervention that was designed around the [https://www.livestronger.org.nz/assets/Uploads/acc1162-otago-exercise-manual.pdf Otago Home Exercise programme]. This protocol targeted [[falls]] prevention, lower limb and [[Core Strengthening|core]] strength exercises with dynamic balance retraining. Their goal was to maximize walking and functional movements required for maintenance and recovery. Patients were prescribed an individualized exercise plan to do three times as well as 30 minute walks twice weekly for a total of 6 weeks. The physiotherapist was able to check in with participants during the 6 weeks. The results demonstrated that a tailored exercise program had a positive effect on a participant’s walking speed and balance in patients with CIDP. More studies will be needed to be able to completely determine the long term impact, however, these are very positive results and should be taken into account when treating a patient with CIDP<ref name=":0" />. | ||
[[File:3-3-2.jpg | [[File:3-3-2.jpg|200x200px|<ref>SCIFIT Upper Body Ergometer - Rent Fitness Equipment [Internet]. Rent Fitness Equipment. 2018 [cited 7 May 2018]. Available from: <nowiki>https://rentfitnessequipment.com/catalogue/scifit-upper-body-ergometer/</nowiki></ref>|right|frameless]] | ||
Markvandsen et al, (2017)<ref name=":10">Markvardsen L, Overgaard K, Heje K, Sindrup S, Christiansen I, Vissing J et al. Resistance training and aerobic training improve muscle strength and aerobic capacity in chronic inflammatory demyelinating polyneuropathy. Muscle & Nerve. 2017;57(1):70-76</ref> examined the effects of a 12 week aerobic or resistance exercise training programs. The aerobic exercise program involved patients on an ergometer bicycle at home or at a local fitness center for 20-30 minutes 3 times a week. The resistance program, which trained the knee and elbow flexors/extensors on the patient’s weaker side, was completed 3 times a week under the supervision of the authors and each exercise was done repeated 12 times in sets of 3. | Markvandsen et al, (2017)<ref name=":10">Markvardsen L, Overgaard K, Heje K, Sindrup S, Christiansen I, Vissing J et al. Resistance training and aerobic training improve muscle strength and aerobic capacity in chronic inflammatory demyelinating polyneuropathy. Muscle & Nerve. 2017;57(1):70-76</ref> examined the effects of a 12 week aerobic or resistance exercise training programs. The aerobic exercise program involved patients on an ergometer bicycle at home or at a local fitness center for 20-30 minutes 3 times a week. The resistance program, which trained the knee and elbow flexors/extensors on the patient’s weaker side, was completed 3 times a week under the supervision of the authors and each exercise was done repeated 12 times in sets of 3. | ||
During the aerobic training period, VO2 max, 6MWT scores and muscle strength increased. Resistance training also resulted in an increase in muscle strength on the trained, initially weaker, side. The results from this study demonstrate that physical exercise training in patients with CIDP is feasible and effective. Their research determined there was no change in disability, quality of life fatigue severity. The authors hypothesised the lack of change in quality of life was due to the difficulty of 3 times a week exercise and exhaustion following exercise<ref name=":10" />. This must be considered when designing an exercise program for someone with CIDP. | During the aerobic training period, VO2 max, 6MWT scores and muscle strength increased. Resistance training also resulted in an increase in muscle strength on the trained, initially weaker, side. The results from this study demonstrate that physical exercise training in patients with CIDP is feasible and effective. Their research determined there was no change in disability, quality of life fatigue severity. The authors hypothesised the lack of change in quality of life was due to the difficulty of 3 times a week exercise and exhaustion following exercise<ref name=":10" />. This must be considered when designing an exercise program for someone with CIDP. | ||
=== Prognosis === | |||
The prognosis for CIDP is highly dependent on age at onset, clinical form of the disease, and initial response to treatment<ref name=":8" />. Although not yet validated, it is suggested that increased time between the onset of symptoms and beginning of treatment will result in poor prognosis<ref name=":8" />. For two thirds of people diagnosed, their disease is progressive while the remaining one third have relapsing episodes<ref name=":5" />. The prognosis is good for patients with monophasic or relapsing course of CIDP, and younger patients with rapid onset or a monophasic course are more likely to respond to treatment<ref name=":8" />. Long term remissions after treatment is less frequent in the elderly population compared to juvenile patients or adults aged younger than 64 years<ref name=":8" />. | The prognosis for CIDP is highly dependent on age at onset, clinical form of the disease, and initial response to treatment<ref name=":8" />. Although not yet validated, it is suggested that increased time between the onset of symptoms and beginning of treatment will result in poor prognosis<ref name=":8" />. For two thirds of people diagnosed, their disease is progressive while the remaining one third have relapsing episodes<ref name=":5" />. The prognosis is good for patients with monophasic or relapsing course of CIDP, and younger patients with rapid onset or a monophasic course are more likely to respond to treatment<ref name=":8" />. Long term remissions after treatment is less frequent in the elderly population compared to juvenile patients or adults aged younger than 64 years<ref name=":8" />. | ||
== | == Outcome Measures == | ||
The following outcome measures have been validated for use in individuals with CIDP | The following outcome measures have been validated for use in individuals with CIDP: | ||
* | * [[Timed Up and Go Test (TUG)|Timed up and go test (TUG)]] → Time taken to stand up from a chair, walk a short distance, turn around, return, and sit down again | ||
* [[10 Metre Walk Test|10-Meter Walk Test (10MWT)]] → walking speed | |||
* [[Grip Strength|Grip strength]] | |||
* [[Muscle Strength Testing|Manual muscle strength testing]] and isokinetic strength testing → muscle strength testing | |||
* [[Fatigue Severity Scale|Fatigue severity scale (FSS)]] → measures fatigue | |||
* [[36-Item Short Form Survey (SF-36)|SF-36]] → quality of life measure; Physical functioning, role functioning social functioning, body pain,mental health, vitality, general health perception, and change in health | |||
* [ | |||
* | |||
* [ | |||
* [ | |||
* [ | |||
* [[Berg Balance Scale|BERG balance scale]] → measures balance during the performance of functional tasks | |||
* [[Visual Analogue Scale|Visual analogue scale (VAS)]] | |||
*[[Six Minute Walk Test / 6 Minute Walk Test|6 Minute walk test (6MWT)]] → walking speed<ref name=":0" /><ref name=":10" /><ref>Allan J, Gelinas D, Lewis R, Nowak R, Wolfe G. Optimizing the Use of Outcome Measures in Chronic Inflammatory Demyelinating Polyneuropathy. US Neurology. 2018;13(1):26-34</ref> | |||
== References == | |||
== | |||
[[Category:Queen's University Neuromotor Function Project]] | [[Category:Queen's University Neuromotor Function Project]] | ||
__FORCETOC__ | __FORCETOC__ | ||
[[Category:Neurology]] | [[Category:Neurology]] | ||
<references /> | |||
[[Category:Neuropathy]] | |||
[[Category:Autoimmune Disorders]] | |||
[[Category:Conditions]] | |||
[[Category:Neurological - Conditions]] | |||
Latest revision as of 12:30, 13 March 2023
Top Contributors - Sydney Cudmore, Lucinda hampton, Megan Grubb, Kim Jackson, Caitlyn Zavitz, Amanda Ager, Aminat Abolade and Tania Bhogal
Introduction[edit | edit source]
Chronic inflammatory demyelinating polyneuropathy (CIDP) is an acquired demyelinating disease involving peripheral nerves, and is generally considered the chronic counterpart to Guillain-Barré syndrome (GBS). About 16% of the patients present with acute GBS.
Patients typically present with a gradual and protracted (>2 months) symmetrical weakness, balance problems, impaired sensation and diminished reflexes and sensory changes.
- The long term nature of this condition leads to abnormalities in gait and impairments in both psychological and social functioning.
- Prognosis is variable and depends on age, clinical course, responsiveness to treatment, and electrophysiological findings. [1][2] [3][4]
This is a helpful 3.5 minute video.
Pathophysiology[edit | edit source]
Chronic inflammatory demyelinating polyneuropathy is an acquired demyelinating disease of the peripheral nervous system.
Surrounding peripheral nerves is a white, fatty layer of myelin that is made up of glial cells, termed Scwhann cells. The myelin coating generated by the Schwann cells assists the axons in transmitting faster neural impulses to receptor sites, such as organs and muscles.
- In CIDP this myelin around the nerves is destroyed resulting in changes of denervation in the supplied muscles.
- Affected nerves demonstrate segmental infiltration with inflammatory cells (lymphocytes) and demyelination.
- With time there is proliferation of Schwann cells and deposition of collagen, which causes thickening of the nerve and a characteristic onion bulb appearance. [1][7]
Etiology[edit | edit source]
CIDP is an autoimmune disorder.
- It involves both T cell-mediated and humoral immune mechanisms by targeting the myelin of the peripheral nervous system.
- Classical CIDP is idiopathic, but it has variants that can be seen in association with a neoplastic process, HIV infections, and chronic history of diabetes mellitus type II.[4]
Epidemiology[edit | edit source]
Overall prevalence has been reported around 0.8 to 8.9 per 100,000 and increases with advancing age (peak incidence of 40 to 60 years of age). CIDP predominantly affects males more than females with a ratio of 2:1 CIDP is suspected to be vastly underestimated, in part due to underreporting, uncertainty in making the diagnosis and diagnostic criteria inconsistencies.[4] [3]
Clinical Presentation[edit | edit source]
Individuals often report difficulty with walking, climbing stairs, balance and manual dexterity, which is attributed to the progressive, symmetric limb weakness and sensory loss. It is commonly seen to affect proximal muscles in the early stages and then progressively move to the distal limbs. Numbing, buzzing and tingling may be characteristic of sensory loss reported by patients and neuropathic pain is less often reported[8].
On the contrary, some individuals may not present with these symptoms, which makes diagnosis much more difficult and can take many years, leaving patients with no answers and increased frustration[9].
After complete subjective and objective history in addition to further medical investigations, which may take multiple months after symptom onset, the following aspects have been recognized as major cardinal features of CIDP[10]:
- Progression over at least 2 months
- Predominant motor symptoms
- Symmetric involvement of arms and legs
- Proximal muscles involved along with distal muscles
- Deep tendon reflexes reduction or absence
- Cerebrospinal fluid (CSF) protein elevation without pleocytosis
- Nerve conduction evidence of a primary demyelinating neuropathy
Differential Diagnosis[edit | edit source]
Differential diagnoses include:[11][12]
- Guillain-Barre syndrome (GBS): acute presentation of CIDP can be similar; difficult to differentiate in the first 6 weeks; after 6-8 weeks GBS should be improving whereas CIDP will demonstrate chronic inflammation. [1]
- Multifocal motor neuropathy
- Diabetic polyneuropathy
- Fibromyalgia
- Multiple sclerosis
- Amyotrophic lateral sclerosis (ALS)
- Neuropathy associated with: nutritional deficiencies, critical illness, toxins, systemic inflammatory diseases.
Diagnosis[edit | edit source]
The diagnosis and management of CIDP is complex and requires an interprofessional inpatient and outpatient team that includes a general neurologist, neuromuscular specialist, intensivist, physical and rehabilitation specialist, pain management specialist, physical therapist, occupational therapist, psychiatrist, social workers, and case management staff [4]
It is not uncommon for CIDP to go undiagnosed for months to years depending on a patient's symptoms. This could be due to symptoms not being prominent enough to affect an individual's day to day routine and cause concern to visit their physician. In addition, the presence of non debilitating symptoms makes it difficult to give a definitive diagnosis of CIDP in the early stages, even after follow up with their physician[9].
Management/Interventions[edit | edit source]
Treatment options for CIDP include corticosteroids, intravenous immunoglobulins (IVIG), and plasma exchange (PLEX). Corticosteroids are used as bridge therapy due to long term side effects. Serial IVIG and plasmapheresis are the mainstays of therapy. Patients who respond to treatment must continue until their condition is stabilized or maximum improvement occurs. Improvements are measured with comparable signs such as improvement in sensation, strength and the performance of activities of daily living (ADLs). Further treatment/maintenance therapy is provided to assist the individual with the intention to reduce the frequency of relapses and slow disease progression. Treatments also aim to reduce patient symptoms, such as weakness and pain, and improve overall functional status. [4][12][8].
Occupational or physiotherapists can prescribe gait aids, specialized tools and ankle-foot Introduction to Orthotics, if necessary, to assist individuals with functional tools for ADL’s and ambulation.
Psychologists may also be an important part of the interprofessional team as they can address symptoms that often accompany a diagnosis of a chronic disease and change in functional status, such as depression, anxiety or frustration[8].
The following describes the process of choosing medical management as suggested by Vallat[13]:
Physiotherapy Management[edit | edit source]
A physiotherapist's role in the treatment of CIDP needs to be very specialized to each individual. Physiotherapists prescribe gait aids that may assist with balance and ambulation during ADL’s. Manual therapy may also be provided when indicated to prevent joint contractures and maintain available ROM. Since many individuals with CIDP have difficulty with balance and walking, gait retraining and exercise programs prove to be beneficial at increasing efficiency and endurance[8].
Exercises that promote muscle strengthening and aerobic conditioning are important once patients have received clearance from a physician for an exercise program[16]. Exercise parameters for CIDP patients also need to be individually tailored as it is important that individuals don’t over-exert themselves. As a result of the demyelination of axons the body is unable to recruit as many muscle fibres to complete a task, which may cause the muscle fibres that are engaged to be overworked. Some delayed onset muscle soreness is expected to occur, but pain that persists longer than 12-48 hours, with or without a loss of strength, is an indication that the F.I.T.T. parameters need to be adjusted because the patient was overexerted[16]. It also crucial to assess that CIDP patients can complete motion against gravity before applying external resistance to the movement, as it is important to have baseline muscle control before progressing exercises[16].
Recent studies on the effects of exercise programs for patients with CIDP have provided good insight and direction for future physiotherapy treatments. Janssen et al, (2018)[2] completed an intervention that was designed around the Otago Home Exercise programme. This protocol targeted falls prevention, lower limb and core strength exercises with dynamic balance retraining. Their goal was to maximize walking and functional movements required for maintenance and recovery. Patients were prescribed an individualized exercise plan to do three times as well as 30 minute walks twice weekly for a total of 6 weeks. The physiotherapist was able to check in with participants during the 6 weeks. The results demonstrated that a tailored exercise program had a positive effect on a participant’s walking speed and balance in patients with CIDP. More studies will be needed to be able to completely determine the long term impact, however, these are very positive results and should be taken into account when treating a patient with CIDP[2].
Markvandsen et al, (2017)[18] examined the effects of a 12 week aerobic or resistance exercise training programs. The aerobic exercise program involved patients on an ergometer bicycle at home or at a local fitness center for 20-30 minutes 3 times a week. The resistance program, which trained the knee and elbow flexors/extensors on the patient’s weaker side, was completed 3 times a week under the supervision of the authors and each exercise was done repeated 12 times in sets of 3.
During the aerobic training period, VO2 max, 6MWT scores and muscle strength increased. Resistance training also resulted in an increase in muscle strength on the trained, initially weaker, side. The results from this study demonstrate that physical exercise training in patients with CIDP is feasible and effective. Their research determined there was no change in disability, quality of life fatigue severity. The authors hypothesised the lack of change in quality of life was due to the difficulty of 3 times a week exercise and exhaustion following exercise[18]. This must be considered when designing an exercise program for someone with CIDP.
Prognosis[edit | edit source]
The prognosis for CIDP is highly dependent on age at onset, clinical form of the disease, and initial response to treatment[13]. Although not yet validated, it is suggested that increased time between the onset of symptoms and beginning of treatment will result in poor prognosis[13]. For two thirds of people diagnosed, their disease is progressive while the remaining one third have relapsing episodes[8]. The prognosis is good for patients with monophasic or relapsing course of CIDP, and younger patients with rapid onset or a monophasic course are more likely to respond to treatment[13]. Long term remissions after treatment is less frequent in the elderly population compared to juvenile patients or adults aged younger than 64 years[13].
Outcome Measures[edit | edit source]
The following outcome measures have been validated for use in individuals with CIDP:
- Timed up and go test (TUG) → Time taken to stand up from a chair, walk a short distance, turn around, return, and sit down again
- 10-Meter Walk Test (10MWT) → walking speed
- Grip strength
- Manual muscle strength testing and isokinetic strength testing → muscle strength testing
- Fatigue severity scale (FSS) → measures fatigue
- SF-36 → quality of life measure; Physical functioning, role functioning social functioning, body pain,mental health, vitality, general health perception, and change in health
- BERG balance scale → measures balance during the performance of functional tasks
- Visual analogue scale (VAS)
- 6 Minute walk test (6MWT) → walking speed[2][18][19]
References[edit | edit source]
- ↑ 1.0 1.1 1.2 Radiopedia Chronic inflammatory demyelinating polyneuropathy Available:https://radiopaedia.org/articles/chronic-inflammatory-demyelinating-polyneuropathy (accessed 23.9.2022)
- ↑ 2.0 2.1 2.2 2.3 Janssen J, Bunce M, Nixon J, Dunbar M, Jones S, Benstead J et al. A clinical case series investigating the effectiveness of an exercise intervention in chronic inflammatory demyelinating polyneuropathy. Physiotherapy Practice and Research. 2018;39(1):37-44
- ↑ 3.0 3.1 Latov N. Diagnosis of CIDP. Neurology. 2002;59(6):S2-S6.
- ↑ 4.0 4.1 4.2 4.3 4.4 Gogia B, Rai PK. Chronic inflammatory demyelinating polyradiculoneuropathy. Available:https://www.statpearls.com/ArticleLibrary/viewarticle/119994 (accessed 23.9.2022)
- ↑ GBS-CIDP Foundation International . Chronic Inflammatory Demyelinating Polyneuropathy (CIDP) 101 . Available from:https://www.youtube.com/watch?v=WJVVYk1sg0I [last accessed 23.9.2022]
- ↑ Purpose of Schwann Cells. (2018). [image] Available at: https://socratic.org/questions/in-human-anatomy-what-is-the-purpose-of-the-schwann-cells [Accessed 7 May 2018].
- ↑ Blumenfeld H. Neuroanatomy through clinical cases. 2nd ed. Sunderland, MA: Sinauer Associates, Inc.; 2010
- ↑ 8.0 8.1 8.2 8.3 8.4 Gorson K. An update on the management of chronic inflammatory demyelinating polyneuropathy. Therapeutic Advances in Neurological Disorders. 2012;5(6):359-373
- ↑ 9.0 9.1 Wolfe, G. and Greer, M. (2015). Diagnosing and Treating CIDP - GBS/CIDP Foundation International. [online] GBS/CIDP Foundation International. Available at: https://www.gbs-cidp.org/ecomm/diagnosing-and-treating-cidp/ [Accessed 7 May 2018].
- ↑ Lewis R. Chronic inflammatory demyelinating polyneuropathy. Current Opinion in Neurology. 2017;30(5):508-512
- ↑ Lewis R. Chronic Inflammatory Demyelinating Polyneuropathy. Neurol Clin. 2007;25(1):71-87. doi:10.1016/j.ncl.2006.11.003.
- ↑ 12.0 12.1 Koller H, Kieseier B, Jander S, Hartung H. Chronic Inflammatory Demyelinating Polyneuropathy. The New England Journal of Medicine. 2005;352:1343-1356
- ↑ 13.0 13.1 13.2 13.3 13.4 Vallat J-M, Sommer C, Magy L. Chronic inflammatory demyelinating polyradiculoneuropathy: diagnostic and therapeutic challenges for a treatable condition. The Lancet Neurology. 2010;9(4):402–12
- ↑ Vallat J-M, Sommer C, Magy L. Chronic inflammatory demyelinating polyradiculoneuropathy: diagnostic and therapeutic challenges for a treatable condition. The Lancet Neurology. 2010;9(4):402–12
- ↑ Manual Therapy – Foul Bay Physio [Internet]. Foulbayphysio.com. 2018 [cited 7 May 2018]. Available from: http://www.foulbayphysio.com/service/manual-therapy/
- ↑ 16.0 16.1 16.2 Hansen M. Exercise for CIDP. 2010
- ↑ SCIFIT Upper Body Ergometer - Rent Fitness Equipment [Internet]. Rent Fitness Equipment. 2018 [cited 7 May 2018]. Available from: https://rentfitnessequipment.com/catalogue/scifit-upper-body-ergometer/
- ↑ 18.0 18.1 18.2 Markvardsen L, Overgaard K, Heje K, Sindrup S, Christiansen I, Vissing J et al. Resistance training and aerobic training improve muscle strength and aerobic capacity in chronic inflammatory demyelinating polyneuropathy. Muscle & Nerve. 2017;57(1):70-76
- ↑ Allan J, Gelinas D, Lewis R, Nowak R, Wolfe G. Optimizing the Use of Outcome Measures in Chronic Inflammatory Demyelinating Polyneuropathy. US Neurology. 2018;13(1):26-34
![[17]](/images/thumb/a/aa/3-3-2.jpg/200px-3-3-2.jpg)






