COPD (Chronic Obstructive Pulmonary Disease)
Original Editor - Rachael Lowe
Top Contributors - Admin, Laura Ritchie, Vidya Acharya, Kim Jackson, Lucinda hampton, Rachael Lowe, Scott Buxton, Shaimaa Eldib, Evan Thomas, Aminat Abolade, George Prudden, Ewa Jaraczewska, WikiSysop, Lauren Lopez, Tony Lowe, Rishika Babburu, Sheik Abdul Khadir, Karen Wilson, Nicole Hills and 127.0.0.1
Introduction[edit | edit source]
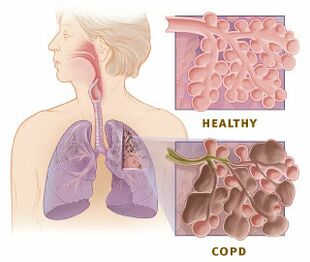
Chronic obstructive pulmonary disease (COPD) is a common and treatable disease characterized by progressive airflow limitation and tissue destruction. It is associated with structural lung changes due to chronic inflammation from prolonged exposure to noxious particles or gases most commonly cigarette smoke. Chronic inflammation causes airway narrowing and decreased lung recoil. The disease often presents with symptoms of cough, dyspnea, and sputum production. Symptoms can range from being asymptomatic to respiratory failure.[1]
Epidemiology[edit | edit source]
COPD is primarily present in smokers and those greater than age 40. Prevalence increases with age and it is currently the third most common cause of morbidity and mortality worldwide. In 2015, the prevalence of COPD was 174 million and there were approximately 3.2 million deaths due to COPD worldwide. However, the prevalence is likely to be underestimated due to the underdiagnosis of COPD.[1]
Etiology[edit | edit source]
COPD is caused by prolonged exposure to harmful particles or gases.
- Cigarette smoking is the most common cause of COPD worldwide.
- Other causes may include second-hand smoke, environmental and occupational exposures, and alpha-1 antitrypsin deficiency (AATD)[1].
Mechanism of Injury / Pathological Process[edit | edit source]
COPD is an inflammatory condition involving the airways, lung parenchyma, and pulmonary vasculature.[1] Emphysema describes one of the structural changes seen in COPD where there is destruction of the alveolar air sacs (gas-exchanging surfaces of the lungs) leading to obstructive physiology.
The process is thought to involve oxidative stress and protease-antiprotease imbalances. In emphysema, an irritant (e.g., smoking) causes an inflammatory response. Neutrophils and macrophages are recruited and release multiple inflammatory mediators. Oxidants and excess proteases lead to the destruction of the air sacs. The protease-mediated destruction of elastin leads to a loss of elastic recoil and results in airway collapse during exhalation.
- The inflammatory response and obstruction of the airways cause a decrease in the forced expiratory volume (FEV1) and tissue destruction leads to airflow limitation and impaired gas exchange.
- Hyperinflation of the lungs is often seen on imaging studies and occurs due to air trapping from airway collapse during exhalation.
- The inability to fully exhale also causes elevations in carbon dioxide (CO2) levels.
- With disease progression the reduction in ventilation or increase in physiologic dead space leads to CO2 retention.
- Acute exacerbations of COPD are common and usually occur due to a trigger (e.g., bacterial or viral pneumonia, environmental irritants). There is an increase in inflammation and air trapping often requiring corticosteroid and bronchodilator treatment.[1]
Clinical Presentation[edit | edit source]
COPD will typically present in adulthood and often during the winter months. Patients usually present with complaints of chronic and progressive dyspnea, cough, and sputum production. Patients may also have wheezing and chest tightness. While a smoking history is present in most cases, there are many without such history. They should be questioned on exposure to second-hand smoke, occupational and environmental exposures, and family history.
COPD is a complex interaction between asthma, chronic bronchitis, and emphysema.
Diagnostic Procedures[edit | edit source]
Unfortunately, there is no single diagnostic test for COPD; diagnosis relies on the presence/absence of symptoms and clinical judgement. The best approach is to undertake a detailed subjective history and physical examination. As COPD is not curable the earlier that it is diagnosed, the earlier treatment can start and that may help to slow down the progression of the disease and the subsequent damage to the lungs.
- Assessment - A diagnosis of COPD should be considered in patients over the age of 35 who have a risk factor (generally smoking) and who present with exertional breathlessness, chronic cough, regular sputum production, frequent winter ‘bronchitis’ or wheeze.
- Spirometry - The presence of airflow obstruction should be confirmed by performing post-bronchodilator spirometry. All health professionals involved in the care of people with COPD should have access to spirometry and be competent in the interpretation of the results
- X-Ray - An x-ray of the chest may show an over-expanded lung (hyperinflation) and can be useful to help exclude other lung diseases.
- Pulmonary function tests - Complete pulmonary function tests with measurements of lung volumes and gas transfer may also show hyperinflation and can discriminate between COPD with emphysema and COPD without emphysema.
- Blood tests - A blood sample taken from an artery can be tested for blood gas levels which may show low oxygen levels (hypoxemia) and/or high carbon dioxide levels (respiratory acidosis). A blood sample taken from a vein may show a high blood count (reactive polycythemia), a reaction to long-term hypoxemia.
Outcome Measures[edit | edit source]
Follow the link here for a list of outcome measures within Physio-pedia.
There can be a different number of ways of measuring the impact or change of someone's COPD, examples being from lung function, lung volumes and exercise capacity. A cross-sectional study recommends cardiopulmonary exercise testing (CPET) as an efficient tool in assessing functional capacity and prognosis in Heart Failure and COPD patients[2].
Lung Function - Forced Expiratory Volume in 1 Second (FEV1)[edit | edit source]
It is known that COPD lungs lose function quicker and more rapidly than non-COPD lungs. In recognition of this FEV1 is the most important marker to determine severity and treatment in COPD algorithms, with decline of FEV1 over-time as the marker for disease progression[3]. The ratio of FEV1/FVC (Forced vital capacity) as well as the percentage predicted FEV1 is a fixed ratio used in current guidelines to assess the function of lungs.
The strengths of using this measure is that:
- FEV1 and FVC measurements are highly reproducible
- Poor lung function if a risk factor for all cause of cardiovascular mortality and poorer health[4]
Limitations being:
- FEV1 measurements are based on an artificial manoeuvre and do not always correlate with clinically relevant outcomes such as dyspnoea, health status, exercise capacity, or exacerbations[5][6]
- Patients with similar FEV1 may represent different underlying phenotypes.
- Reference equations for lung function by European Community for Coal and Steel are disputed and limited in predicting lung function in the general population [7]
- No minimal important difference (MID) has been established yet. It was suggested that an appropriate range of values for the MID for FEV1 might be 100-140 mL but the MID for FEV1 remains poorly defined for COPD [8]
Maximum voluntary ventilation(MVV)[edit | edit source]
The maximal voluntary ventilation (MVV) is the maximum amount of air inhaled and then exhaled during a 12 to 15 seconds interval with maximal voluntary effort. MMV provides information about the functioning of the inspiratory pump, chest wall, maximum ventilatory capacity and respiratory muscle endurance. The assessment of MVV is also used as a target for respiratory muscle training with normocapnic hyperpnea modality. A retrospective study in healthy people and COPD patients concludes that MVV measurement should be carried out directly instead of estimating through prediction as the values of the actual maximum voluntary ventilation MVV estimated from equations are scattered and may underestimate or overestimate the real MVV value in these populations; so estimated results should not be used as a replacement for the real value of MVV[9].
Lung Volumes[edit | edit source]
Changes in absolute lung volumes can occur in COPD patients even in the absence of FEV1 changes. Progressive hyperinflation due to airflow limitation and loss of lung elastic recoil not only increases the work required during inspiration but also profoundly decreases the ventilatory reserve and increases the sense of effort and dyspnoea[10]
In terms of measurement static lung hyperinflation and its increase during exercise (dynamic hyperinflation) are measured as elevations of total lung capacity (TLC), functional residual capacity (FRC), residual volume (RV) and as a decrease in inspiratory capacity (IC)[3].
Strengths of lung volumes include:
- Indices of dynamic hyperinflation correlate better than FEV1 with activity limitation and exertional dyspnoeaand pharmacological and surgical lung volume reduction have been associated with improvements in exercise performance and dyspnoea[11][12]
- A severely reduced IC/TLC ratio with a threshold value of 25% has been shown to predict mortality in COPD patients[13]
Weaknesses include:
- Body plethysmography remains the gold standard for the measurement of lung volumes such as TLC, FRC and RV. Spirometrically derived assessments of lung hyperinflation are more difficult to interpret in the absence of simultaneous bodyplethysmographic volume measurements to rule out a concomitant restrictive ventilatory disorder[14]
- The reproducibility of FRC, IC and RV in absolute values has yet to be demonstrated. Measurement of IC alone is not a reliable marker of lung hyperinflation and does not consistently reflect changes in FRC or TLC[14]
- The natural course of dynamic hyperinflation in COPD is unknown and seems likely to be highly variable among COPD patients [14]
Exercise Capacity[edit | edit source]
Includes the 6 Minute Walk test, the Bleep Test, Shuttle Walk Test and Ergometry.
Management / Interventions[edit | edit source]
As COPD is not curable the aim of treatment and interventions are directed at improving quality of life by managing symptoms and exacerbations and slowing down damage to the lungs. According to a longitudinal study[15], changes in frailty status of COPD patients were associated with significant clinical outcomes related to dyspnea, mobility, physical activity, and handgrip and quadriceps strength. It was found that five-times sit-to-stand (5STS) and exacerbations were independent predictors of the improvement in frailty status.
Smoking Cessation[edit | edit source]
Encouraging patients with COPD to stop smoking is one of the most important components of their management. All COPD patients still smoking, regardless of age, should be encouraged to stop, and offered help to do so, at every opportunity.
Exercise[edit | edit source]
| [16] |
Exercise prescription is a key component of pulmonary rehabilitation programmes, which are part of the non-pharmacological approach to managing COPD. There is a high level of evidence for the benefits of pulmonary rehabilitation for people with COPD[17] Strength and endurance exercise are endorsed for people with COPD.[18]
Muscles that are required for arm exercise are also involved in movement of the chest wall during respiration and thus the need to breathe often compromises the individual’s ability to undertake daily activities, therefore exercise prescription involving arm exercise needs to be carefully prescribed.[19]
Promote Effective Inhaled Therapy[edit | edit source]
In people with stable COPD who remain breathless or have exacerbations despite use of short-acting bronchodilators as required, offer the following as maintenance therapy:
- if forced expiratory volume in 1 second (FEV1)≥50% predicted: either long-acting beta2 agonist (LABA) or long-acting muscarinic antagonist (LAMA)
- if FEV1<50% predicted: either LABA with an inhaled corticosteroid (ICS) in a combination inhaler, or LAMA
Offer LAMA in addition to LABA + ICS to people with COPD who remain breathless or have exacerbations despite taking LABA + ICS, irrespective of their FEV1.
Provide Pulmonary Rehabilitation[edit | edit source]
Pulmonary rehabilitation (PR) should be made available to all appropriate people with COPD including those who have had a recent hospitalisation for an acute exacerbation. A randomised study suggests positive outcomes with functional electrostimulation in patients with severe chronic obstructive pulmonary disease hospitalized for acute exacerbation[20]. A study suggests that patients affected with COPD and pulmonary hypertension experience a lower exercise capacity and quality of life[21]. Another randomized controlled trial examining the effects of virtual training (VR) and exercise training on the rehabilitation of patients with COPD suggests that pulmonary rehabilitation program supplemented with VR training has positive outcomes in improving physical fitness in patients with COPD[22]. Studies suggest PR was useful in patients with moderate to severe COPD[23]. A prospective, multisite, randomised controlled trial will determine whether an 8-week PR programme (exercise training will comprise: overground or treadmill walking, lower limb stationary cycling, lower and upper limb strengthening exercises) is equivalent to a 12-week PR programme in people with COPD[24].
Use Non-Invasive Ventilation[edit | edit source]
| [25] |
Non-invasive ventilation (NIV) should be used as the treatment of choice for persistent hypercapnic ventilatory failure during exacerbations not responding to medical therapy. It should be delivered by staff trained in its application, experienced in its use and aware of its limitations. When patients are started on NIV, there should be a clear plan covering what to do in the event of deterioration and ceilings of therapy should be agreed.
Manage Exacerbations[edit | edit source]
The frequency of exacerbations should be reduced by appropriate use of inhaled corticosteroids and bronchodilators, and vaccinations.
The impact of exacerbations should be minimised by:
- Giving self-management advice on responding promptly to the symptoms of an exacerbation
- Starting appropriate treatment with oral steroids and/or antibiotics
- Use of non-invasive ventilation when indicated
- Use of hospital-at-home or assisted-discharge schemes
Utilise a Multidisciplinary Team[edit | edit source]
COPD care should be delivered by a multidisciplinary team.
Managing Symptoms and Conditions in Stable COPD[edit | edit source]
Breathlessness and Exacerbations[edit | edit source]
- Manage breathlessness and exercise limitation with inhaled therapy
- For exacerbations or persistent breathlessness:
- Use long-acting bronchodilators or LABA + ICS
- Consider adding theophylline if still symptomatic
- Offer pulmonary rehabilitation to all suitable people. An observational study suggests pulmonary rehabilitation significantly improves hospital days and emergency department presentations in the first 12 months post-program. Healthcare utilization benefits are less evident in the second 12 months[26].
- Refer patients who are breathless, have a single large bulla on a CT scan and an FEV1 less than 50% predicted for consideration of bullectomy
- Refer people with severe COPD for consideration of lung volume reduction surgery if they remain breathless with marked restrictions of their activities of daily living, despite maximal medical therapy (including rehabilitation), and meet all of the following:
- FEV1 greater than 20% predicted
- PaCO2 less than 7.3 kPa
- Upper lobe predominant emphysema
- TLCO greater than 20% predicted
- Consider referring people with severe COPD for assessment for lung transplantation if they remain breathless with marked restrictions of their activities of daily living despite maximal medical therapy. Considerations include:
- Age
- FEV1
- PaCO2
- Homogeneously distributed emphysema on CT scan
- Elevated pulmonary artery pressures with progressive deterioration
- Comorbidities
- Local surgical protocols
Frequent Exacerbations[edit | edit source]
- Optimise inhaled therapy
- Offer vaccinations and prophylaxis
- Give self-management advice
- Consider osteoporosis prophylaxis for people requiring frequent oral corticosteroids
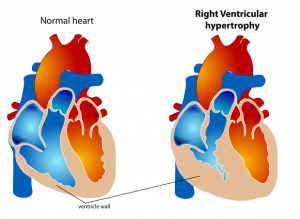
Cor Pulmonale[edit | edit source]
- Consider in people who have peripheral edema, a raised venous pressure, a systolic parasternal heave, a loud pulmonary second heart sound
- Exclude other causes of peripheral edema
- Perform pulse oximetry, ECG and echocardiogram if features of cor pulmonale
- Assess need for LTOT
- Treat edema with diuretic
- Angiotensin-converting enzyme inhibitors, calcium channel blockers, alpha-blockers are not recommended
- Digoxin may be used where there is atrial fibrillation
Respiratory Failure[edit | edit source]
- Assess for appropriate oxygen
- Consider referral for assessment for long-term domiciliary NIV therapy
Abnormal BMI[edit | edit source]
- Refer for dietetic advice
- Offer nutritional supplements if the BMI is low
- Pay attention to weight changes in older patients (especially>3 kg)
Chronic Productive Cough[edit | edit source]
- Consider mucolytic therapy
- A single-arm pilot study analyzing the impact of a specific Oscillating positive expiratory pressure (oPEP) - Aerobika® device in COPD patients' lung dynamics and drug deposition suggests that the Aerobika® device usage led to an improved airflow causing a shift in internal airflow distribution and impacted the drug deposition patterns of the medication in patients with COPD[27].
Anxiety and Depression[edit | edit source]
- Screen for anxiety and depression using validated tools in people who are:
- Hypoxic
- Severely breathless or
- Have recently been seen or treated at a hospital for an exacerbation
- Refer to NICE guidelines ‘Depression with a chronic physical health problem.’[28]
Alpha-1 Antitrypsin Deficiency[edit | edit source]
- Offer referral to a specialist centre to discuss the clinical management of this condition
- Alpha-1 antitrypsin replacement therapy is not recommended
Palliative Setting[edit | edit source]
- Opioids should be used when appropriate for the palliation of breathlessness in people with end-stage COPD unresponsive to other medical therapy
- Use benzodiazepines, tricyclic antidepressants, major tranquillisers and oxygen to treat breathlessness
- Provide access to multidisciplinary palliative care teams and hospices
Resources[edit | edit source]
- KNGF guidelines for physical therapy in patients with chronic obstructive pulmonary disease
- Mesothelioma Resources for Patients
Videos[edit | edit source]
References[edit | edit source]
- ↑ 1.0 1.1 1.2 1.3 1.4 Agarwal AK, Raja A, Brown BD. Chronic obstructive pulmonary disease (COPD). StatPearls [Internet]. 2020 Jun 7.Available from:https://www.ncbi.nlm.nih.gov/books/NBK559281/ (accessed 24.5.2021)
- ↑ da Luz GC, Rossi CF, Tinoco AG, Marinho RS, de Faria CP, da Silva AT, Oliveira CR, Borghi-Silva A, Mendes RG, Goi RM. The Value of Cardiopulmonary Exercise Testing in Determining Severity in Patients with both Systolic Heart Failure and COPD. Scientific Reports (Nature Publisher Group). 2020 Dec 1;10(1).
- ↑ 3.0 3.1 Glaab T. Vogelmeier C and Buhl R. Outcome measures in chronic obstructive pulmonary disease (COPD): strengths and limitations. Respiratory Research. 2010:11:79
- ↑ Sin DD, Wu L, Man SF: The relationship between reduced lung function and cardiovascular mortality: a population-based study and a systematic review of the literature. Chest 2005, 127:1952-1959
- ↑ Cazzola M, MacNee W, Martinez FJ, Rabe KF, Franciosi LG, Barnes PJ, Brusasco V, Burge PS, Calverley PMA, Celli BR, Jones PW, Mahler DA, Make B, Miravitlles M, Page CP, Palange P, Parr D, Pistolesi M, Rennard SI, Rutten-van Mölken MP, Stockley R, Sullivan SD, Wedzicha JA, Wouters EF, American Thoracic Society/European Respiratory Society Task Force on outcomes of COPD: Outcomes for COPD pharmacological trials: from lung function to biomarkers. Eur Respir J 2008, 31:416-469
- ↑ 11.Wise RA: The value of forced expiratory volume in 1 second decline in the assessment of chronic obstructive pulmonary disease progression. Am J Med 2006, 119:4-11
- ↑ 12.Pellegrino R, Viegi G, Brusasco V, Crapo RO, Burgos F, Casaburi R, Coates A, van der Grinten CPM, Gustafsson P, Hankinson J, Jensen R, Johnson DC, MacIntyre N, McKay R, Miller MR, Navajas D, Pedersen OF, Wanger J: Interpretative strategies for lung function tests. Eur Respir J 2005, 26:948-968
- ↑ 4.Cazzola M, MacNee W, Martinez FJ, Rabe KF, Franciosi LG, Barnes PJ, Brusasco V, Burge PS, Calverley PMA, Celli BR, Jones PW, Mahler DA, Make B, Miravitlles M, Page CP, Palange P, Parr D, Pistolesi M, Rennard SI, Rutten-van Mölken MP, Stockley R, Sullivan SD, Wedzicha JA, Wouters EF, American Thoracic Society/European Respiratory Society Task Force on outcomes of COPD: Outcomes for COPD pharmacological trials: from lung function to biomarkers. Eur Respir J 2008, 31:416-469
- ↑ Otto-Yáñez M, Sarmento A, Torres-Castro R, Russelly P, Carvalho de Farias C, Dornelas De Andrade AD, Puppo H, Resqueti V, Fregonezi GA. Maximal Voluntary Ventilation Should Not Be Estimated From the Forced Expiratory Volume in the First Second in Healthy People and COPD Patients. Frontiers in Physiology. 2020;11:537.
- ↑ O'Donnell DE, Laveneziana P: Physiology and consequences of lung hyperinflation in COPD. Eur Respir Rev 2006, 15:61-67
- ↑ 17.O'Donnell DE: Is sustained pharmacologic lung volume reduction now possible in COPD? Chest 2006, 129:501-503
- ↑ 18.Criner GJ, Belt P, Sternberg AL, Mosenifar Z, Make BJ, Utz JP, Sciurba F: National Emphysema Treatment Trial Research Group. Effects of lung volume reduction surgery on gas exchange and breathing pattern during maximum exercise. Chest 2009, 135:1268-79
- ↑ 19.Casanova C, Cote C, de Torres JP, Aguirre-Jaime A, Marin JM, Pinto-Plata V, Celli BR: Inspiratory-to-total lung capacity predicts mortality in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2005, 171:591-597
- ↑ 14.0 14.1 14.2 O'Donnell DE, Laveneziana P: Physiology and consequences of lung hyperinflation in COPD. Eur Respir Rev 2006, 15:61-67
- ↑ Bernabeu-Mora R, Oliveira-Sousa SL, Sánchez-Martínez MP, García-Vidal JA, Gacto-Sánchez M, Medina-Mirapeix F. Frailty transitions and associated clinical outcomes in patients with stable COPD: A longitudinal study. Plos one. 2020 Apr 3;15(4):e0230116.
- ↑ Burke Rehabilitation. COPD Treatments & Rehab: Upper Body Exercises. Available from: http://www.youtube.com/watch?v=VR7QnSnHmBU[last accessed 13/02/15]
- ↑ Roisin RR, Rabe KF, Anzueto A, et al. Global strategy for the diagnosis management, and prevention of chronic obstructive pulmonary disease. Bethesda, MD: Global Initiative for Chronic Obstructive Lung Disease, 2008; 1–91.
- ↑ Skinner, Margot. Strength and endurance exercise endorsed for people with COPD. Physical Therapy Reviews, Volume 14, Number 6, December 2009 , pp. 418-418(1)
- ↑ Ennis S, Alison J, McKeough Z. The effects of arm endurance and strength training on arm exercise capacity in people with chronic obstructive pulmonary disease. Phys Ther Rev 2009;14(4):226–39.
- ↑ Lopez-Lopez L, Torres-Sanchez I, Rodriguez-Torres J, Cabrera-Martos I, Cahalin LP, Valenza MC. Randomized feasibility study of twice a day functional electrostimulation in patients with severe chronic obstructive pulmonary disease hospitalized for acute exacerbation. Physiotherapy theory and practice. 2019 Nov 23:1-8.
- ↑ Blanco I, Valeiro B, Torres-Castro R, Barberán-García A, Torralba Y, Moisés J, Sebastián L, Osorio J, Rios J, Gimeno-Santos E, Roca J.[Effects of Pulmonary Hypertension on Exercise Capacity in Patients With Chronic Obstructive Pulmonary Disease. Archivos de bronconeumologia. 2019 Nov 23.
- ↑ Rutkowski S, Rutkowska A, Kiper P, Jastrzebski D, Racheniuk H, Turolla A, Szczegielniak J, Casaburi R. Virtual Reality Rehabilitation in Patients with Chronic Obstructive Pulmonary Disease: A Randomized Controlled Trial. International Journal of Chronic Obstructive Pulmonary Disease. 2020;15:117.
- ↑ Lee AL, Butler SJ, Varadi RG, Goldstein RS, Brooks D. The Impact of Pulmonary Rehabilitation on Chronic Pain in People with COPD. COPD: Journal of Chronic Obstructive Pulmonary Disease. 2020 Mar 3:1-0.
- ↑ Bishop J, Spencer L, Alison J. Effect of a pulmonary rehabilitation programme of 8 weeks compared to 12 weeks duration on exercise capacity in people with chronic obstructive pulmonary disease (PuRe Duration): protocol for a randomised controlled trial. BMJ open respiratory research. 2020 Sep 1;7(1):e000687.
- ↑ SMACC. Non-Invasive Ventilation. Available from: http://www.youtube.com/watch?v=QQZvhkBWBgQ [last accessed 13/02/15]
- ↑ Walsh JR, Pegg J, Yerkovich ST, Morris N, McKeough ZJ, Comans T, Paratz JD, Chambers DC. Longevity of pulmonary rehabilitation benefit for chronic obstructive pulmonary disease—health care utilisation in the subsequent 2 years. BMJ Open Respiratory Research. 2019 Nov 1;6(1).
- ↑ Leemans G, Belmans D, Van Holsbeke C, Kushnarev V, Sugget J, Ides K, Vissers D, De Backer W. A Functional Respiratory Imaging Approach to the Effect of an Oscillating Positive Expiratory Pressure Device in Chronic Obstructive Pulmonary Disease. International Journal of Chronic Obstructive Pulmonary Disease. 2020 Jun 4;15:1261-8.
- ↑ National Institute for Health and Clinical Excellence. Chronic obstructive pulmonary disease: Management of chronic obstructive pulmonary disease in adults in primary and secondary care. Available from http://guidance.nice.org.uk/CG91 [last accessed 2/8/10]