COPD (Chronic Obstructive Pulmonary Disease): Difference between revisions
No edit summary |
mNo edit summary |
||
| (19 intermediate revisions by 4 users not shown) | |||
| Line 7: | Line 7: | ||
== Introduction == | == Introduction == | ||
[[File:Copd versus healthy lung.jpeg|right|frameless|310x310px]] | [[File:Copd versus healthy lung.jpeg|right|frameless|310x310px]] | ||
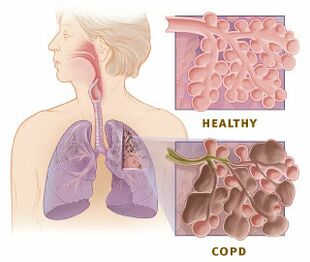
Chronic obstructive pulmonary disease (COPD) is a common and treatable disease | Chronic obstructive pulmonary disease (COPD)<ref name=":1">Hillas G, Perlikos F, Tsiligianni I, Tzanakis N. Managing comorbidities in COPD. International journal of chronic obstructive pulmonary disease. 2015 Jan 7:95-109.</ref> is a common and treatable disease characterised by progressive airflow limitation and tissue destruction. It is associated with structural [[Lung Anatomy|lung]] changes due to chronic [[Inflammation Acute and Chronic|inflammation]] from prolonged exposure to noxious particles or gases, most commonly cigarette smoke. Chronic inflammation causes airway narrowing and decreased lung recoil. The disease often presents with cough symptoms, [[Dyspnoea|dyspnea]], and sputum production. Symptoms can range from being asymptomatic to [[Respiratory Failure|respiratory failure]].<ref name=":0">Agarwal AK, Raja A, Brown BD. Chronic obstructive pulmonary disease (COPD). StatPearls [Internet]. 2020 Jun 7.Available from:https://www.ncbi.nlm.nih.gov/books/NBK559281/ (accessed 24.5.2021)</ref> | ||
== Epidemiology == | == Epidemiology == | ||
COPD is primarily present in smokers and those | COPD is primarily present in [[Smoking Cessation and Brief Intervention|smokers]] and those over 40. Prevalence increases with age and is currently the third most common cause of morbidity and mortality worldwide. In 2015, the prevalence of COPD was 174 million, and there were approximately 3.2 million deaths due to COPD worldwide. However, the prevalence is likely to be underestimated due to the under diagnosis of COPD.<ref name=":0" /> | ||
== Etiology == | == Etiology == | ||
COPD is caused by prolonged exposure to harmful particles or gases. | COPD is caused by prolonged exposure to harmful particles or gases. | ||
* Cigarette smoking is the most common cause of COPD worldwide. | * Cigarette smoking is the most common cause of COPD worldwide.<ref name=":1" /> | ||
* Other causes may include second-hand smoke, environmental and occupational exposures, | * Other causes may include second-hand smoke, environmental and occupational exposures,alpha-1 antitrypsin deficiency (AATD)<ref name=":0" /> ageing <ref name=":2">Agustí A, Vogelmeier C, Faner R. COPD 2020: changes and challenges. American Journal of Physiology-Lung Cellular and Molecular Physiology. 2020 Nov 1;319(5):L879-83.</ref>and gene-environment interactions (GxE)<ref name=":2" /> . | ||
{| width="100%" cellspacing="1" cellpadding="1" | {| width="100%" cellspacing="1" cellpadding="1" | ||
|- | |- | ||
| {{#ev:youtube|T1G9Rl65M-Q|412}} | | {{#ev:youtube|T1G9Rl65M-Q|412}} <ref>Animated COPD Patient. Understanding COPD. Available from: https://www.youtube.com/watch?v=T1G9Rl65M-Q [last accessed 31/5/2022]</ref> | ||
| | | | ||
|} | |} | ||
== Mechanism of Injury / Pathological Process == | == Mechanism of Injury / Pathological Process == | ||
[[File:Healthy alveoli Primal.png|right|frameless|399x399px]] | |||
COPD is an inflammatory condition involving the airways, [[Lung Anatomy|lung]] parenchyma, and pulmonary vasculature.<ref name=":0" /> [[Emphysema]] describes one of the structural changes seen in COPD where there is the destruction of the [[Alveoli|alveolar]] air sacs (gas-exchanging surfaces of the lungs) leading to obstructive physiology. | |||
Image 2: Healthy Alveoli. | |||
The process is thought to involve [[Free Radicals|oxidative stress]] and protease-antiprotease imbalances. In emphysema, an irritant (e.g., smoking) causes an [[Inflammation Acute and Chronic|inflammatory]] response. Neutrophils and macrophages are recruited and release multiple inflammatory mediators. Oxidants and excess proteases lead to the destruction of the air sacs. The protease-mediated destruction of elastin leads to a loss of elastic recoil and results in airway collapse during exhalation. | The process is thought to involve [[Free Radicals|oxidative stress]] and protease-antiprotease imbalances. In emphysema, an irritant (e.g., smoking) causes an [[Inflammation Acute and Chronic|inflammatory]] response. Neutrophils and macrophages are recruited and release multiple inflammatory mediators. Oxidants and excess proteases lead to the destruction of the air sacs. The protease-mediated destruction of elastin leads to a loss of elastic recoil and results in airway collapse during exhalation. | ||
* The inflammatory response and obstruction of the airways cause a decrease in the [[Spirometry|forced expiratory volume]] (FEV1) and tissue destruction leads to airflow limitation and impaired gas exchange. | * The inflammatory response and obstruction of the airways cause a decrease in the [[Spirometry|forced expiratory volume]] (FEV1), and tissue destruction leads to airflow limitation and impaired gas exchange. | ||
* Hyperinflation of the lungs is often seen | * Hyperinflation of the lungs is often seen in imaging studies and occurs due to air trapping from airway collapse during exhalation. | ||
* The inability to fully | * The inability to exhale fully also causes elevations in carbon dioxide (CO2) levels. | ||
* With disease progression the reduction in ventilation or increase in physiologic dead space leads to CO2 retention. | * With disease progression the reduction in [[Ventilation and Weaning|ventilation]] or increase in '''physiologic dead space''' leads to CO2 retention. | ||
* Acute exacerbations of COPD are common and usually occur due to a trigger (e.g., [[Bacterial Infections|bacterial]] or [[Viral Infections|viral]] [[pneumonia]], environmental irritants). There is an increase in inflammation and air trapping often requiring [[Corticosteroid Medication|corticosteroid]] and bronchodilator treatment.<ref name=":0" /> | * Acute exacerbations of COPD are common and usually occur due to a trigger (e.g., [[Bacterial Infections|bacterial]] or [[Viral Infections|viral]] [[pneumonia]], environmental irritants). There is an increase in inflammation and air trapping, often requiring [[Corticosteroid Medication|corticosteroid]] and bronchodilator treatment.<ref name=":0" /> | ||
== Clinical Presentation == | == Clinical Presentation == | ||
COPD will typically present in adulthood and often during the winter months. Patients usually present with complaints of chronic and progressive [[Dyspnoea|dyspnea]], cough, and sputum production. Patients may also have wheezing and chest tightness. While a smoking history is present in most cases, there are many without such history. They should be questioned on exposure to second-hand smoke, occupational and [[An Introduction to Environmental Physiotherapy|environmental]] exposures, and family history. | [[File:Dyspnea 01.png|right|frameless|399x399px]] | ||
COPD will typically present in adulthood and often during the winter months. Patients usually present with complaints of chronic and progressive [[Dyspnoea|dyspnea]], cough, and sputum production. Patients may also have wheezing and chest tightness. While a smoking history is present in most cases, there are many without such a history. They should be questioned on exposure to second-hand smoke, occupational and [[An Introduction to Environmental Physiotherapy|environmental]] exposures, and family history.<ref name=":1" /><ref name=":2" /> | |||
COPD is a complex interaction between [[asthma]], [[Chronic Bronchitis|chronic bronchitis]], and [[emphysema]]. | COPD is a complex interaction between [[asthma]], [[Chronic Bronchitis|chronic bronchitis]], and [[emphysema]]. | ||
== | == Evaluation == | ||
[[File:Spirometry1.jpg|right|frameless]] | |||
*Assessment - A diagnosis of COPD should be considered in patients over the age of 35 who have a risk factor (generally smoking) and who present with exertional breathlessness, chronic cough, regular sputum production, frequent winter | COPD is often evaluated in patients with relevant symptoms and risk factors. The diagnosis is confirmed by [[spirometry]]. Other tests may include a [[Six Minute Walk Test / 6 Minute Walk Test|6-minute walk test]], laboratory testing, and [[Medical Imaging|radiographic imaging.]] | ||
*Assessment - A diagnosis of COPD should be considered in patients over the age of 35 who have a risk factor (generally smoking) and who present with exertional breathlessness, chronic cough, regular sputum production, frequent winter ‘[[bronchitis]]’ or wheeze. | |||
*X-Ray - An | *X-Ray - An X-ray of the chest may show an over-expanded lung (hyperinflation) and can be useful to help exclude other lung diseases. | ||
*[[Pulmonary Function Test|Pulmonary function tests]] - | *[[Pulmonary Function Test|Pulmonary function tests]] - Essential in the diagnosis, staging, and monitoring of COPD. [[Spirometry]] is performed before and after administering an inhaled bronchodilator. Inhaled bronchodilators may be short-acting beta2-agonist (SABA), short-acting anticholinergic, or a combination of both. A ratio of the forced expiratory volume in one second to forced vital capacity (FEV1/FVC) less than 0.7 confirms the diagnosis of COPD. Patients with significantly reduced FEV1 and signs of dyspnea should be evaluated for oxygenation with pulse [[Oxygen Therapy|oximetry]] or [[Arterial Blood Gases|arterial blood gas]] analysis. | ||
*[[Blood Tests|Blood tests]] - A blood sample taken from an artery can be tested for [[Arterial Blood Gases|blood gas levels]] which may show low oxygen levels (hypoxemia) and/or high carbon dioxide levels (respiratory acidosis). A blood sample taken from a vein may show a high blood count (reactive polycythemia), a reaction to long-term hypoxemia. | *[[Blood Tests|Blood tests]] - A blood sample taken from an artery can be tested for [[Arterial Blood Gases|blood gas levels]] which may show low oxygen levels ([[Hypoxaemia|hypoxemia]]) and/or high carbon dioxide levels ([[Respiratory Failure|respiratory acidosis]]). A blood sample taken from a vein may show a high [[Blood Physiology|blood]] count (reactive polycythemia), a reaction to long-term hypoxemia. | ||
{| width="100%" cellspacing="1" cellpadding="1" | {| width="100%" cellspacing="1" cellpadding="1" | ||
|- | |- | ||
| {{#ev:youtube|YwcNbVnHNAo|412}} | | {{#ev:youtube|YwcNbVnHNAo|412}} <ref>Armando Hasudungan. Understanding Spirometry - Normal, Obstructive vs Restrictive. Available from: https://www.youtube.com/watch?v=YwcNbVnHNAo [last accessed 31/5/2022]</ref> | ||
| {{#ev:youtube|s8pXdtp_Duw|412}} | | {{#ev:youtube|s8pXdtp_Duw|412}} <ref>nhswestminster. Spirometry Procedure. Available from: https://www.youtube.com/watch?v=s8pXdtp_Duw [last accessed 31/5/2022]</ref> | ||
|} | |} | ||
== Outcome Measures == | == Outcome Measures == | ||
[[File:Oximetre.jpg|right|frameless]] | |||
There can be a different number of ways of measuring the impact or change of someone's COPD, examples being lung function, [[Lung Volumes|lung volumes]] and [[Therapeutic Exercise|exercise]] capacity. A cross-sectional study recommends [[Cardiopulmonary Exercise Testing (CPET) In Adults|cardiopulmonary exercise testing]] (CPET) as an efficient tool in assessing functional capacity and prognosis in Heart Failure and COPD patients<ref>da Luz GC, Rossi CF, Tinoco AG, Marinho RS, de Faria CP, da Silva AT, Oliveira CR, Borghi-Silva A, Mendes RG, Goi RM. [https://pubmed.ncbi.nlm.nih.gov/32152432/ The Value of Cardiopulmonary Exercise Testing in Determining Severity in Patients with Systolic Heart Failure and COPD]. Scientific Reports (Nature Publisher Group). 2020 Dec 1;10(1).</ref>. | |||
Other [[Outcome Measures|outcome measures]] include: | |||
Bleep Test; Shuttle Walk Test; Ergometry; [[Borg Rating Of Perceived Exertion|BORG RPE]]; [[Six Minute Walk Test / 6 Minute Walk Test|6-minute walk test]] is commonly performed to assess the submaximal functional capacity of a patient. This test is performed indoors on a flat and straight surface. The length of the hallway is usually 100 feet, and the test measures the distance the patient walks over a period of 6 minutes<ref name=":0" />; [[Grip Strength]]; [[30 Seconds Sit To Stand Test|30 seconds Sit to stand]] | |||
According to a longitudinal study<ref>Bernabeu-Mora R, Oliveira-Sousa SL, Sánchez-Martínez MP, García-Vidal JA, Gacto-Sánchez M, Medina-Mirapeix F. [https://pubmed.ncbi.nlm.nih.gov/32243447/ Frailty transitions and associated clinical outcomes in patients with stable COPD: A longitudinal study.] Plos one. 2020 Apr 3;15(4):e0230116.</ref>, changes in [[Introduction to Frailty|frailty]] status of COPD patients were associated with significant clinical outcomes related to dyspnea; mobility; [[Physical Activity|physical activity]]; handgrip and [[Quadriceps Muscle|quadriceps]] strength. It was found that five times sit-to-stand and exacerbations were independent predictors of the improvement in frailty status. | |||
== Management / Interventions == | |||
[[File:Smoking man.jpg|right|frameless]] | |||
The primary goals of treatment are to control symptoms, improve the [[Quality of Life|quality of life]], and reduce exacerbations and mortality. The non-pharmacological approach includes [[Smoking Cessation and Brief Intervention|smoking cessation]] and [[Pulmonary Rehabilitation| Pulmonary rehabilitation]]. | |||
Annual [[influenza]] [[Vaccines|vaccination]] is recommended in all patients with COPD. | |||
The classes of commonly used medications in COPD include: | |||
* Bronchodilators (beta2-agonists, antimuscarinics, methylxanthines), | |||
* Inhaled [[Corticosteroid Medication|corticosteroids]] (ICS) and systemic glucocorticoids, | |||
* Phosphodiesterase-4 (PDE4) inhibitors, | |||
* [[Antibiotics|Antibiotics.]] | |||
=== Exercise === | === Exercise === | ||
| Line 109: | Line 85: | ||
|} | |} | ||
Exercise prescription is a key component of pulmonary rehabilitation programmes, | Exercise prescription is a key component of pulmonary rehabilitation programmes, part of the non-pharmacological approach to managing COPD. There is a high level of evidence for the benefits of pulmonary rehabilitation for people with COPD<ref>Roisin RR, Rabe KF, Anzueto A, et al. Global strategy for diagnosing, managing, and preventing chronic obstructive pulmonary disease. Bethesda, MD: Global Initiative for Chronic Obstructive Lung Disease, 2008; 1–91.</ref> [[Strength Training|Strength]] and endurance exercises are endorsed for people with COPD.<ref>Skinner, Margot. Strength and endurance exercises are endorsed for people with COPD. Physical Therapy Reviews, Volume 14, Number 6, December 2009, pp. 418-418(1)</ref> | ||
Use of [[Exercise and Protein Supplements|protein supplements]], in combination with exercise, could also be beneficial. Refer to the dietician. | |||
Muscles that are required for arm exercise are also involved in movement of the chest wall during respiration and thus the need to breathe often compromises the individual’s ability to undertake daily activities, | Muscles that are required for arm exercise are also involved in the movement of the chest wall during respiration, and thus, the need to breathe often compromises the individual’s ability to undertake daily activities. Therefore, exercise prescription involving arm exercise needs to be carefully prescribed.<ref>Ennis S, Alison J, McKeough Z. The effects of arm endurance and strength training on arm exercise capacity in people with chronic obstructive pulmonary disease. Phys Ther Rev 2009;14(4):226–39.</ref> The evidence showed that patients with COPD have limb muscle dysfunction, a key systemic consequence.<ref>Spruit MA, Singh SJ, Garvey C, ZuWallack R, Nici L, Rochester C, Hill K, Holland AE, Lareau SC, Man WD, Pitta F. An official American Thoracic Society/European Respiratory Society statement: key concepts and advances in pulmonary rehabilitation. American Journal of Respiratory and critical care medicine. 2013 Oct 15;188(8):e13-64.</ref> | ||
=== Promote Effective Inhaled Therapy === | === Promote Effective Inhaled Therapy === | ||
In people with stable COPD who remain breathless or have exacerbations despite use of short-acting bronchodilators as required, offer the following as maintenance therapy: | In people with stable COPD who remain breathless or have exacerbations despite the use of short-acting bronchodilators as required, offer the following as maintenance therapy: | ||
*if forced expiratory volume in 1 second (FEV1)≥50% predicted: either long-acting beta2 agonist (LABA) or long-acting muscarinic antagonist (LAMA) | *if forced expiratory volume in 1 second (FEV1)≥50% predicted: either long-acting beta2 agonist (LABA) or long-acting muscarinic antagonist (LAMA) | ||
*if FEV1<50% predicted: either LABA with an inhaled corticosteroid (ICS) in a combination inhaler | *if FEV1<50% predicted: either LABA with an inhaled corticosteroid (ICS) in a combination inhaler or LAMA | ||
Offer LAMA in addition to LABA + ICS to people with COPD who remain breathless or have exacerbations despite taking LABA + ICS, irrespective of their FEV1. | Offer LAMA in addition to LABA + ICS to people with COPD who remain breathless or have exacerbations despite taking LABA + ICS, irrespective of their FEV1. | ||
=== Provide Pulmonary Rehabilitation === | === Provide Pulmonary Rehabilitation === | ||
[[File:Exercise older person.jpg|right|frameless]] | |||
[[Pulmonary Rehabilitation|Pulmonary rehabilitation]] (PR) should be | [[Pulmonary Rehabilitation|Pulmonary rehabilitation]] (PR) <ref>Holland AE, Cox NS, Houchen-Wolloff L, Rochester CL, Garvey C, ZuWallack R, Nici L, Limberg T, Lareau SC, Yawn BP, Galwicki M. Defining modern pulmonary rehabilitation. An official American Thoracic Society workshop report. Annals of the American Thoracic Society. 2021 May;18(5):e12-29.</ref>should be available to all appropriate people with COPD, including those with a recent hospitalisation for an acute exacerbation. A randomised study suggests positive outcomes with functional electrostimulation in patients with severe chronic obstructive pulmonary disease hospitalised for acute exacerbation<ref>Lopez-Lopez L, Torres-Sanchez I, Rodriguez-Torres J, Cabrera-Martos I, Cahalin LP, Valenza MC. [https://pubmed.ncbi.nlm.nih.gov/31769337-randomized-feasibility-study-of-twice-a-day-functional-electrostimulation-in-patients-with-severe-chronic-obstructive-pulmonary-disease-hospitalized-for-acute-exacerbation/ Randomized feasibility study of twice a day functional electrostimulation in patients with severe chronic obstructive pulmonary disease hospitalized for acute exacerbation.] Physiotherapy theory and practice. 2019 Nov 23:1-8.</ref>. A study suggests that patients affected with COPD and pulmonary hypertension experience a lower exercise capacity and quality of life<ref>Blanco I, Valeiro B, Torres-Castro R, Barberán-García A, Torralba Y, Moisés J, Sebastián L, Osorio J, Rios J, Gimeno-Santos E, Roca J.[Effects of Pulmonary Hypertension on Exercise Capacity in Patients With Chronic Obstructive Pulmonary Disease. Archivos de bronconeumologia. 2019 Nov 23.</ref>. Another randomised controlled trial examining the effects of virtual training (VR) and exercise training on the rehabilitation of patients with COPD suggests that pulmonary rehabilitation program supplemented with VR training has positive outcomes in improving physical fitness in patients with COPD<ref>Rutkowski S, Rutkowska A, Kiper P, Jastrzebski D, Racheniuk H, Turolla A, Szczegielniak J, Casaburi R. [https://pubmed.ncbi.nlm.nih.gov/32021150-virtual-reality-rehabilitation-in-patients-with-chronic-obstructive-pulmonary-disease-a-randomized-controlled-trial/ Virtual Reality Rehabilitation in Patients with Chronic Obstructive Pulmonary Disease: A Randomized Controlled Trial.] International Journal of Chronic Obstructive Pulmonary Disease. 2020;15:117.</ref>. Studies suggest PR was useful in patients with moderate to severe COPD<ref>Lee AL, Butler SJ, Varadi RG, Goldstein RS, Brooks D. [https://pubmed.ncbi.nlm.nih.gov/32131643/ The Impact of Pulmonary Rehabilitation on Chronic Pain in People with COPD.] COPD: Journal of Chronic Obstructive Pulmonary Disease. 2020 Mar 3:1-0.</ref>. A prospective, multisite, randomised controlled trial will determine whether an 8-week PR programme (exercise training will comprise: overground or treadmill walking, lower limb stationary cycling, and lower and upper limb strengthening exercises) is equivalent to a 12-week PR programme in people with COPD<ref>Bishop J, Spencer L, Alison J. [https://pubmed.ncbi.nlm.nih.gov/32933927/ Effect of a pulmonary rehabilitation programme of 8 weeks compared to 12 weeks duration on exercise capacity in people with chronic obstructive pulmonary disease (PuRe Duration): protocol for a randomised controlled trial]. BMJ open respiratory research. 2020 Sep 1;7(1):e000687.</ref>. | ||
=== Utilise a Multidisciplinary Team === | === Utilise a Multidisciplinary Team === | ||
A multidisciplinary team should deliver COPD care. | |||
=== Palliative Setting === | === Palliative Setting === | ||
*Opioids should be used when appropriate for the palliation of breathlessness in people with end-stage COPD unresponsive to other medical therapy | *[[Opioids]] should be used when appropriate for the palliation of breathlessness in people with end-stage COPD unresponsive to other medical therapy | ||
*Use benzodiazepines, tricyclic antidepressants, major tranquillisers and oxygen to treat breathlessness | *Use benzodiazepines, tricyclic antidepressants, major tranquillisers and oxygen to treat breathlessness | ||
*Provide access to multidisciplinary palliative care teams and hospices | *Provide access to multidisciplinary palliative care teams and hospices | ||
| Line 231: | Line 116: | ||
*[[Clinical Guidelines: Cardiopumlonary#COPD|Clinical Guidelines for COPD]] | *[[Clinical Guidelines: Cardiopumlonary#COPD|Clinical Guidelines for COPD]] | ||
*[https://www.mesothelioma.com/ Mesothelioma Resources for Patients] | *[https://www.mesothelioma.com/ Mesothelioma Resources for Patients] | ||
| Line 239: | Line 122: | ||
{| class="FCK__ShowTableBorders" cellspacing="1" cellpadding="1" width="100%" | {| class="FCK__ShowTableBorders" cellspacing="1" cellpadding="1" width="100%" | ||
|- | |- | ||
| {{#ev:youtube|aktIMBQSXMo|412}} | | {{#ev:youtube|aktIMBQSXMo|412}} <ref>Healthguru. Understanding Chronic Obstructive Pulmonary Disease (COPD#1). Available from: https://www.youtube.com/watch?v=aktIMBQSXMo [last accessed 31/5/2022]</ref> | ||
| {{#ev:youtube|ttdma8PnFJI|412}} | | {{#ev:youtube|ttdma8PnFJI|412}} <ref>Healthguru. Treating Chronic Obstructive Pulmonary Disease (COPD #2). Available from: https://www.youtube.com/watch?v=ttdma8PnFJI [last accessed 31/5/2022]</ref> | ||
|} | |} | ||
Latest revision as of 15:26, 26 June 2023
Original Editor - Rachael Lowe
Top Contributors - Admin, Laura Ritchie, Vidya Acharya, Kim Jackson, Lucinda hampton, Rachael Lowe, Scott Buxton, Shaimaa Eldib, Aminat Abolade, Evan Thomas, George Prudden, Lauren Lopez, Ewa Jaraczewska, WikiSysop, Nicole Hills, 127.0.0.1, Tony Lowe, Rishika Babburu, Sheik Abdul Khadir and Karen Wilson
Introduction[edit | edit source]
Chronic obstructive pulmonary disease (COPD)[1] is a common and treatable disease characterised by progressive airflow limitation and tissue destruction. It is associated with structural lung changes due to chronic inflammation from prolonged exposure to noxious particles or gases, most commonly cigarette smoke. Chronic inflammation causes airway narrowing and decreased lung recoil. The disease often presents with cough symptoms, dyspnea, and sputum production. Symptoms can range from being asymptomatic to respiratory failure.[2]
Epidemiology[edit | edit source]
COPD is primarily present in smokers and those over 40. Prevalence increases with age and is currently the third most common cause of morbidity and mortality worldwide. In 2015, the prevalence of COPD was 174 million, and there were approximately 3.2 million deaths due to COPD worldwide. However, the prevalence is likely to be underestimated due to the under diagnosis of COPD.[2]
Etiology[edit | edit source]
COPD is caused by prolonged exposure to harmful particles or gases.
- Cigarette smoking is the most common cause of COPD worldwide.[1]
- Other causes may include second-hand smoke, environmental and occupational exposures,alpha-1 antitrypsin deficiency (AATD)[2] ageing [3]and gene-environment interactions (GxE)[3] .
| [4] |
Mechanism of Injury / Pathological Process[edit | edit source]
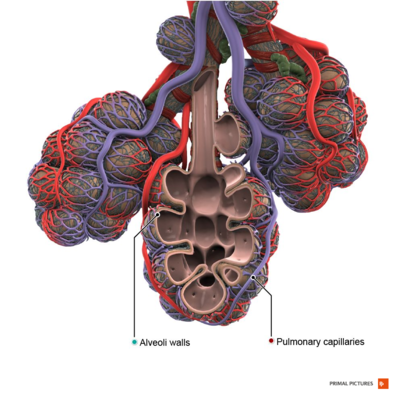
COPD is an inflammatory condition involving the airways, lung parenchyma, and pulmonary vasculature.[2] Emphysema describes one of the structural changes seen in COPD where there is the destruction of the alveolar air sacs (gas-exchanging surfaces of the lungs) leading to obstructive physiology.
Image 2: Healthy Alveoli.
The process is thought to involve oxidative stress and protease-antiprotease imbalances. In emphysema, an irritant (e.g., smoking) causes an inflammatory response. Neutrophils and macrophages are recruited and release multiple inflammatory mediators. Oxidants and excess proteases lead to the destruction of the air sacs. The protease-mediated destruction of elastin leads to a loss of elastic recoil and results in airway collapse during exhalation.
- The inflammatory response and obstruction of the airways cause a decrease in the forced expiratory volume (FEV1), and tissue destruction leads to airflow limitation and impaired gas exchange.
- Hyperinflation of the lungs is often seen in imaging studies and occurs due to air trapping from airway collapse during exhalation.
- The inability to exhale fully also causes elevations in carbon dioxide (CO2) levels.
- With disease progression the reduction in ventilation or increase in physiologic dead space leads to CO2 retention.
- Acute exacerbations of COPD are common and usually occur due to a trigger (e.g., bacterial or viral pneumonia, environmental irritants). There is an increase in inflammation and air trapping, often requiring corticosteroid and bronchodilator treatment.[2]
Clinical Presentation[edit | edit source]
COPD will typically present in adulthood and often during the winter months. Patients usually present with complaints of chronic and progressive dyspnea, cough, and sputum production. Patients may also have wheezing and chest tightness. While a smoking history is present in most cases, there are many without such a history. They should be questioned on exposure to second-hand smoke, occupational and environmental exposures, and family history.[1][3]
COPD is a complex interaction between asthma, chronic bronchitis, and emphysema.
Evaluation[edit | edit source]
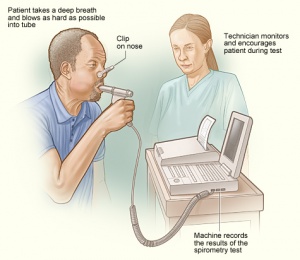
COPD is often evaluated in patients with relevant symptoms and risk factors. The diagnosis is confirmed by spirometry. Other tests may include a 6-minute walk test, laboratory testing, and radiographic imaging.
- Assessment - A diagnosis of COPD should be considered in patients over the age of 35 who have a risk factor (generally smoking) and who present with exertional breathlessness, chronic cough, regular sputum production, frequent winter ‘bronchitis’ or wheeze.
- X-Ray - An X-ray of the chest may show an over-expanded lung (hyperinflation) and can be useful to help exclude other lung diseases.
- Pulmonary function tests - Essential in the diagnosis, staging, and monitoring of COPD. Spirometry is performed before and after administering an inhaled bronchodilator. Inhaled bronchodilators may be short-acting beta2-agonist (SABA), short-acting anticholinergic, or a combination of both. A ratio of the forced expiratory volume in one second to forced vital capacity (FEV1/FVC) less than 0.7 confirms the diagnosis of COPD. Patients with significantly reduced FEV1 and signs of dyspnea should be evaluated for oxygenation with pulse oximetry or arterial blood gas analysis.
- Blood tests - A blood sample taken from an artery can be tested for blood gas levels which may show low oxygen levels (hypoxemia) and/or high carbon dioxide levels (respiratory acidosis). A blood sample taken from a vein may show a high blood count (reactive polycythemia), a reaction to long-term hypoxemia.
| [5] | [6] |
Outcome Measures[edit | edit source]
There can be a different number of ways of measuring the impact or change of someone's COPD, examples being lung function, lung volumes and exercise capacity. A cross-sectional study recommends cardiopulmonary exercise testing (CPET) as an efficient tool in assessing functional capacity and prognosis in Heart Failure and COPD patients[7].
Other outcome measures include:
Bleep Test; Shuttle Walk Test; Ergometry; BORG RPE; 6-minute walk test is commonly performed to assess the submaximal functional capacity of a patient. This test is performed indoors on a flat and straight surface. The length of the hallway is usually 100 feet, and the test measures the distance the patient walks over a period of 6 minutes[2]; Grip Strength; 30 seconds Sit to stand
According to a longitudinal study[8], changes in frailty status of COPD patients were associated with significant clinical outcomes related to dyspnea; mobility; physical activity; handgrip and quadriceps strength. It was found that five times sit-to-stand and exacerbations were independent predictors of the improvement in frailty status.
Management / Interventions[edit | edit source]
The primary goals of treatment are to control symptoms, improve the quality of life, and reduce exacerbations and mortality. The non-pharmacological approach includes smoking cessation and Pulmonary rehabilitation.
Annual influenza vaccination is recommended in all patients with COPD.
The classes of commonly used medications in COPD include:
- Bronchodilators (beta2-agonists, antimuscarinics, methylxanthines),
- Inhaled corticosteroids (ICS) and systemic glucocorticoids,
- Phosphodiesterase-4 (PDE4) inhibitors,
- Antibiotics.
Exercise[edit | edit source]
| [9] |
Exercise prescription is a key component of pulmonary rehabilitation programmes, part of the non-pharmacological approach to managing COPD. There is a high level of evidence for the benefits of pulmonary rehabilitation for people with COPD[10] Strength and endurance exercises are endorsed for people with COPD.[11]
Use of protein supplements, in combination with exercise, could also be beneficial. Refer to the dietician.
Muscles that are required for arm exercise are also involved in the movement of the chest wall during respiration, and thus, the need to breathe often compromises the individual’s ability to undertake daily activities. Therefore, exercise prescription involving arm exercise needs to be carefully prescribed.[12] The evidence showed that patients with COPD have limb muscle dysfunction, a key systemic consequence.[13]
Promote Effective Inhaled Therapy[edit | edit source]
In people with stable COPD who remain breathless or have exacerbations despite the use of short-acting bronchodilators as required, offer the following as maintenance therapy:
- if forced expiratory volume in 1 second (FEV1)≥50% predicted: either long-acting beta2 agonist (LABA) or long-acting muscarinic antagonist (LAMA)
- if FEV1<50% predicted: either LABA with an inhaled corticosteroid (ICS) in a combination inhaler or LAMA
Offer LAMA in addition to LABA + ICS to people with COPD who remain breathless or have exacerbations despite taking LABA + ICS, irrespective of their FEV1.
Provide Pulmonary Rehabilitation[edit | edit source]
Pulmonary rehabilitation (PR) [14]should be available to all appropriate people with COPD, including those with a recent hospitalisation for an acute exacerbation. A randomised study suggests positive outcomes with functional electrostimulation in patients with severe chronic obstructive pulmonary disease hospitalised for acute exacerbation[15]. A study suggests that patients affected with COPD and pulmonary hypertension experience a lower exercise capacity and quality of life[16]. Another randomised controlled trial examining the effects of virtual training (VR) and exercise training on the rehabilitation of patients with COPD suggests that pulmonary rehabilitation program supplemented with VR training has positive outcomes in improving physical fitness in patients with COPD[17]. Studies suggest PR was useful in patients with moderate to severe COPD[18]. A prospective, multisite, randomised controlled trial will determine whether an 8-week PR programme (exercise training will comprise: overground or treadmill walking, lower limb stationary cycling, and lower and upper limb strengthening exercises) is equivalent to a 12-week PR programme in people with COPD[19].
Utilise a Multidisciplinary Team[edit | edit source]
A multidisciplinary team should deliver COPD care.
Palliative Setting[edit | edit source]
- Opioids should be used when appropriate for the palliation of breathlessness in people with end-stage COPD unresponsive to other medical therapy
- Use benzodiazepines, tricyclic antidepressants, major tranquillisers and oxygen to treat breathlessness
- Provide access to multidisciplinary palliative care teams and hospices
Resources[edit | edit source]
Videos[edit | edit source]
| [20] | [21] |
References[edit | edit source]
- ↑ 1.0 1.1 1.2 Hillas G, Perlikos F, Tsiligianni I, Tzanakis N. Managing comorbidities in COPD. International journal of chronic obstructive pulmonary disease. 2015 Jan 7:95-109.
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 Agarwal AK, Raja A, Brown BD. Chronic obstructive pulmonary disease (COPD). StatPearls [Internet]. 2020 Jun 7.Available from:https://www.ncbi.nlm.nih.gov/books/NBK559281/ (accessed 24.5.2021)
- ↑ 3.0 3.1 3.2 Agustí A, Vogelmeier C, Faner R. COPD 2020: changes and challenges. American Journal of Physiology-Lung Cellular and Molecular Physiology. 2020 Nov 1;319(5):L879-83.
- ↑ Animated COPD Patient. Understanding COPD. Available from: https://www.youtube.com/watch?v=T1G9Rl65M-Q [last accessed 31/5/2022]
- ↑ Armando Hasudungan. Understanding Spirometry - Normal, Obstructive vs Restrictive. Available from: https://www.youtube.com/watch?v=YwcNbVnHNAo [last accessed 31/5/2022]
- ↑ nhswestminster. Spirometry Procedure. Available from: https://www.youtube.com/watch?v=s8pXdtp_Duw [last accessed 31/5/2022]
- ↑ da Luz GC, Rossi CF, Tinoco AG, Marinho RS, de Faria CP, da Silva AT, Oliveira CR, Borghi-Silva A, Mendes RG, Goi RM. The Value of Cardiopulmonary Exercise Testing in Determining Severity in Patients with Systolic Heart Failure and COPD. Scientific Reports (Nature Publisher Group). 2020 Dec 1;10(1).
- ↑ Bernabeu-Mora R, Oliveira-Sousa SL, Sánchez-Martínez MP, García-Vidal JA, Gacto-Sánchez M, Medina-Mirapeix F. Frailty transitions and associated clinical outcomes in patients with stable COPD: A longitudinal study. Plos one. 2020 Apr 3;15(4):e0230116.
- ↑ Burke Rehabilitation. COPD Treatments & Rehab: Upper Body Exercises. Available from: http://www.youtube.com/watch?v=VR7QnSnHmBU[last accessed 13/02/15]
- ↑ Roisin RR, Rabe KF, Anzueto A, et al. Global strategy for diagnosing, managing, and preventing chronic obstructive pulmonary disease. Bethesda, MD: Global Initiative for Chronic Obstructive Lung Disease, 2008; 1–91.
- ↑ Skinner, Margot. Strength and endurance exercises are endorsed for people with COPD. Physical Therapy Reviews, Volume 14, Number 6, December 2009, pp. 418-418(1)
- ↑ Ennis S, Alison J, McKeough Z. The effects of arm endurance and strength training on arm exercise capacity in people with chronic obstructive pulmonary disease. Phys Ther Rev 2009;14(4):226–39.
- ↑ Spruit MA, Singh SJ, Garvey C, ZuWallack R, Nici L, Rochester C, Hill K, Holland AE, Lareau SC, Man WD, Pitta F. An official American Thoracic Society/European Respiratory Society statement: key concepts and advances in pulmonary rehabilitation. American Journal of Respiratory and critical care medicine. 2013 Oct 15;188(8):e13-64.
- ↑ Holland AE, Cox NS, Houchen-Wolloff L, Rochester CL, Garvey C, ZuWallack R, Nici L, Limberg T, Lareau SC, Yawn BP, Galwicki M. Defining modern pulmonary rehabilitation. An official American Thoracic Society workshop report. Annals of the American Thoracic Society. 2021 May;18(5):e12-29.
- ↑ Lopez-Lopez L, Torres-Sanchez I, Rodriguez-Torres J, Cabrera-Martos I, Cahalin LP, Valenza MC. Randomized feasibility study of twice a day functional electrostimulation in patients with severe chronic obstructive pulmonary disease hospitalized for acute exacerbation. Physiotherapy theory and practice. 2019 Nov 23:1-8.
- ↑ Blanco I, Valeiro B, Torres-Castro R, Barberán-García A, Torralba Y, Moisés J, Sebastián L, Osorio J, Rios J, Gimeno-Santos E, Roca J.[Effects of Pulmonary Hypertension on Exercise Capacity in Patients With Chronic Obstructive Pulmonary Disease. Archivos de bronconeumologia. 2019 Nov 23.
- ↑ Rutkowski S, Rutkowska A, Kiper P, Jastrzebski D, Racheniuk H, Turolla A, Szczegielniak J, Casaburi R. Virtual Reality Rehabilitation in Patients with Chronic Obstructive Pulmonary Disease: A Randomized Controlled Trial. International Journal of Chronic Obstructive Pulmonary Disease. 2020;15:117.
- ↑ Lee AL, Butler SJ, Varadi RG, Goldstein RS, Brooks D. The Impact of Pulmonary Rehabilitation on Chronic Pain in People with COPD. COPD: Journal of Chronic Obstructive Pulmonary Disease. 2020 Mar 3:1-0.
- ↑ Bishop J, Spencer L, Alison J. Effect of a pulmonary rehabilitation programme of 8 weeks compared to 12 weeks duration on exercise capacity in people with chronic obstructive pulmonary disease (PuRe Duration): protocol for a randomised controlled trial. BMJ open respiratory research. 2020 Sep 1;7(1):e000687.
- ↑ Healthguru. Understanding Chronic Obstructive Pulmonary Disease (COPD#1). Available from: https://www.youtube.com/watch?v=aktIMBQSXMo [last accessed 31/5/2022]
- ↑ Healthguru. Treating Chronic Obstructive Pulmonary Disease (COPD #2). Available from: https://www.youtube.com/watch?v=ttdma8PnFJI [last accessed 31/5/2022]