Breast Cancer
Top Contributors - Adam El-Sayed, Kim Jackson, Lucinda hampton, Vidya Acharya, Admin, Manisha Shrestha, 127.0.0.1, Chee Wee Tan, Ilona Malkauskaite and Candace Goh - Ashlea Anthony & Linsey Schmalz from Bellarmine University.
Introduction[edit | edit source]
Breast cancer is the commonest malignancy in female patients.[1]
- Breast cancer is the most common cancer of women in the United States. As of 2018, 1 in 8 women in the U.S. will have had a diagnosis of invasive breast cancer in their lifetime. This risk has been increasing throughout the years since 1975.[2]
- Globally, female breast cancer is ranked 5th in terms of cancer mortality.[3]
- From 2014-2018, it was found that the average age of women diagnosed with breast cancer is 63 years old.[2]
The management of breast cancer is in constant evolution. Fortunately, survival rates continue to improve, likely due to improved individualized treatment as well as earlier detection[4].
The increase in the number of breast cancer survivors has resulted in more research and care being directed toward developing interventions that will help improve the overall quality of life for women who have survived breast cancer.[5]
- Physiotherapists have an important role in the rehabilitation process during and after a diagnosis of breast cancer, as well as in the care of survivors.
- Physical Activity and physiotherapy treatments has been proven to reduce the incidence of post-cancer musculoskeletal disorders[6].
- Breast cancer involves an interprofessional team to achieve the best possible outcomes. This team includes oncologic and plastic surgeons, medical oncology, radiation oncology, pathology, physiotherapy, radiology, nurse navigators, and multiple other individuals to discuss each patient and formulate a treatment plan. The outcomes for patients with breast cancer continue to improve with the increased use of interprofessional teams, as demonstrated in multiple retrospective studies[4].
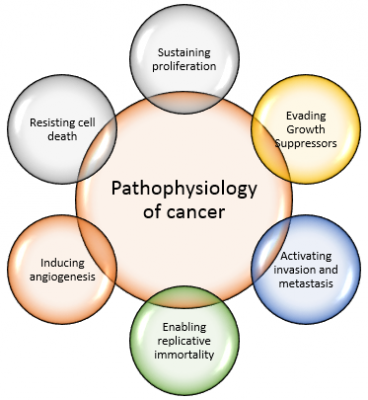
Pathophysiology[edit | edit source]
Breast cancer is a malignant tumor that starts in the cells of the breast. Like other cancers, there are several factors that can raise the risk of getting breast cancer.
- Damage to the DNA and genetic mutations can lead to breast cancer have been experimentally linked to estrogen exposure.
- Some individuals inherit defects in the DNA and genes like the BRCA1, BRCA2 and P53 among others. Those with a family history of ovarian or breast cancer thus are at an increased risk of breast cancer.
- The immune system normally seeks out cancer cells and cells with damaged DNA and destroys them. Breast cancer may be a result of failure of such an effective immune defence and surveillance.
- These are several signalling systems of growth factors and other mediators that interact between stromal cells and epithelial cells. Disrupting these may lead to breast cancer as well[7].
Classification[edit | edit source]
The vast majority of breast cancers are adenocarcinomas (99%). The most common types are:
- Invasive carcinoma of no special type (ductal carcinoma not otherwise specified): 40-75%
- Ductal carcinoma in situ: 20-25% (non invasive, in the ducts or lobules)
- Invasive lobular carcinoma: 5-15%[8]
Terminology
- Grade - “score” on the cancer cells’ appearance and growth patterns: Grade 1 (sometimes also called well differentiated); Grade 2 (moderately differentiated);Grade 3 high grade (poorly differentiated).
- Tumor Necrosis - If present, this means that dead breast cancer cells can be seen within the tissue sample. Tumor necrosis is often limited to a small area within the sample. Its presence suggests a more aggressive breast cancer.
- Vascular or Lymphatic Invasion: - these types of invasion describe whether or not cancerous cells are evident in the vascular and lymphatic vessels supplying the breast tissue.
- Hormone Receptor Status: - Breast cancer cells taken out during a biopsy or surgery are tested to see if they have estrogen or progesterone receptors. When the hormones estrogen and progesterone attach to these receptors, they fuel the cancer growth. Cancers are called hormone receptor-positive or hormone receptor-negative based on whether or not they have these receptors[9]. Hormone receptor status determines if hormone therapy would be appropriate.
- HER2 Status: - HER2 is a gene that when dysfunctional can play a role in the development of breast cancer. Breast cancers that are HER2 positive tend to grow faster and are more likely to spread that those that are HER2 negative.[10]
Stage is the most basic way of categorizing how far a cancer has spread from its point of origin[13]. The stages are the number zero and the Roman numerals I, II, III, or IV (often followed by A, B, or C). In general, the higher the number, the more advanced the cancer. eg Stage IV. Breast cancer cells have spread far away from the breast and lymph nodes right around it. The most common sites are the bones, lungs, liver, and brain. This stage is described as “metastatic,” meaning it has spread beyond the region of the body where it was first found.
Staging of breast tumours uses the TNM system published by the American Joint Committee on Cancer/Union for International Cancer Control (UICC): breast cancer (staging).
The TNM system uses information on:
- T: tumour size and how far it has spread within the breast and nearby organs
- N: lymph node involvement
- M: the presence or absence of distant metastases
Once the T, N, and M are determined through stage grouping, a stage of 0, I, II, III, or IV is assigned.The stage number and degree of cancer spread are positively correlated.
Metastases
Metastasis involves the spread to one or more sites elsewhere in the body. This occurs by way of directly affecting an organ or travelling through the lymphatic and/or circulatory systems.[10]
The following terms can be utilized to classify how far the malignant cells have spread:[14]
- Localized means there is no spread.
- Regional means there is spread to the lymph nodes, tissues, or organs close to where cancer started (the primary site).
- Distant (also known as metastatic cancer) means there is spread to organs or tissues that are farther away from the primary site. The main sites of metastasis for breast cancer include bones, lungs, brain, and liver.[15]
Epidemiology[edit | edit source]
Breast cancer is the most common nonskin malignancy in women.
- In the affluent populations of North America, Europe, and Australia, 6% of women develop invasive breast cancer before age 75, compared to a 2% risk in developing regions of Africa and Asia. The difference has been attributed to risks associated with a Westernized lifestyle, including high calorie diet rich in fat and protein and physical inactivity[8]
- Survivor-ship varies across the globe, such that 5-year relative survival was ≥80% in the United States, Canada, and Austria, but <40% in Denmark, Poland, and Algeria.[16] This may be attributed to differences in diagnostics and treatments, as well as a lack of healthcare resources in some countries[17][18][19]
- Breast cancer-related lymphoedema (BCRL) is condition that a woman can develop anytime 3-20 years after treatment.[20] The incidence varies and likely depends on the type of treatment received. Recent evidence suggests that 1 in 5 women will acquire it at some point.[21]
Risk Factors[edit | edit source]
- increasing age
- reproductive lifestyle factors increasing unopposed oestrogen load
- early menarche
- nulliparity, infertility, or, if parous, few children with late age at first delivery
- lack of breast feeding
- late menopause
- unopposed oestrogen hormone replacement therapy
- personal history of breast cancer or a high risk breast lesion
- first degree relative with breast cancer
- genetic mutations
- BRCA1 or BRCA2 mutation
- Li Fraumeni syndrome
- Peutz Jegher syndrome
- Cowden syndrome
- ataxia telangiectasia
- thoracic radiation therapy
- alcohol consumption[8]
Factors that May Reduce Breast Cancer Risk
- Breastfeeding
- Participating in moderate or vigorous activity
- Maintaining a healthy body weight[22]
Clinical Presentation[edit | edit source]
- Breast cancer may be asymptomatic and undetectable in its earlier stages.
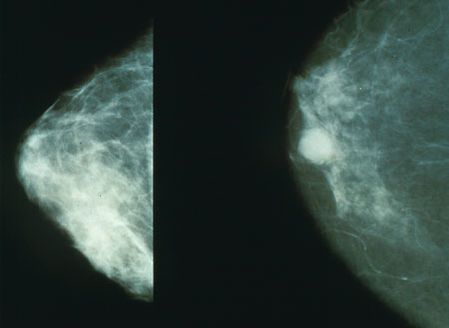
- The hallmark signs and symptoms of a ductal carcinoma are a lump in the breast and breast tenderness (not usually pain).
- The hallmark signs and symptoms of a lobular carcinoma do not involve a lump. Therefore, a lobular carcinoma may be harder to detect
- There is often a change in breast texture.[23]
- Axillary lymph node enlargement or breathlessness (metastases)[1]
Diagnosis[edit | edit source]
- Mammogram (older) and ultrasound (younger)
- Breast MRI for challenging cases
- US/mammogram guided biopsy[1]
- IR thermography: It is a powerful tool that is also non-invasive and non-intrusive easing the analysis, providing safety and comfort to the patients. It can be used in women of different ages and health conditions without any risk[24].
- Hormone Receptor Tests If someone is diagnosed with breast cancer, hormone receptor tests can be used to help develop treatment options. If the cancerous tissue is positive for hormone receptors (estrogen and/or progesterone) then hormone therapy is a recommended form of treatment.[25][26]
- HER2/neu Test: HER2 is the human epidermal growth factor receptor-2, which is a protein that can sometimes be found on cancer cells. The cancer cells that contain the HER2/neu protein tend to be more aggressive and may have a less favourable prognosis. If this is the case, then a targeted approach to that specific area will be used as a treatment option.[25][26][27]
Systemic Involvement[edit | edit source]
Breast cancer that has metastasized can be manifested in several ways[26][28].
- Bone: is the most frequent site of metastasis in both men and women and symptoms can include back hip or shoulder pain, and/or pain with weight-bearing.
- Central Nervous System: is another frequent site for metastasizes of breast cancer, especially at the thoracic levels of the spinal cord. Signs and symptoms that are associated with neurologic involvement include unilateral upper extremity numbness and tingling (cervical/thoracic), leg weakness or paresis (lumbar), or bowel and bladder symptoms (sacral). Other common sites of metastases are lymph nodes, lung, brain, and liver, as well as the remaining breast tissue. Neurologic involvement can also be manifested in a paraneoplastic syndrome, which is a term used to describe associated signs and symptoms at a site that is distant from the tumour and/or metastasis.
- Paraneoplastic syndromes often present in ways that seem uncorrelated with cancer and may mimic disorders of the endocrine, metabolic, hematologic, or neuromuscular systems.
Management[edit | edit source]
see also Oncology Medical Management
Breast cancer often requires surgery as part of curative treatment. In most early-stage breast cancer, surgery is the first step in treatment.
- The decision to proceed with mastectomy or breast conservation surgery remains both patient- and disease-driven. Some patients require upfront chemotherapy and/or radiation treatment to downstage their tumor or axillary nodes, as is the case in inflammatory breast cancer.
- Following surgery, adjuvant radiation is recommended in nearly all patients who undergo breast conservation therapy as recurrence rates are unacceptably high without it.
- Endocrine therapy is recommended for at least five years in those whose tumors are positive for hormone receptors (i.e., estrogen, progesterone) and often recommended for women considered high risk as prophylactic therapy.
- Chemotherapy is also recommended in more aggressive tumors as well as those who have a negative expression of estrogen, progesterone, and HER2neu receptors.[29]
Surgery[edit | edit source]
There are two main types of surgery to remove breast cancer:
- Breast-conserving surgery (also called a lumpectomy, quadrantectomy, partial mastectomy, or segmental mastectomy) is a surgery in which only the part of the breast containing the cancer is removed. The goal is to remove the cancer as well as some surrounding normal tissue. How much breast is removed depends on where and how big the tumor is, as well as other factors.
- Mastectomy is a surgery in which the entire breast is removed, including all of the breast tissue and sometimes other nearby tissues. There are several different types of mastectomies. Some women may also get a double mastectomy, in which both breasts are removed.
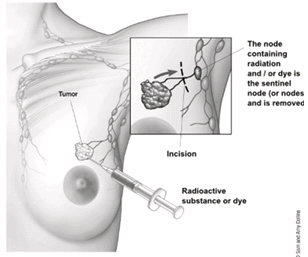
To find out if the breast cancer has spread to underarm (axillary) lymph nodes, one or more of these lymph nodes will be removed and looked at in the lab. Lymph nodes may be removed either as part of the surgery to remove the breast cancer or as a separate operation.The two main types of surgery to remove lymph nodes are:
- Sentinel lymph node biopsy (SLNB) is a procedure in which the surgeon removes only the lymph node(s) under the arm where the cancer would likely spread first. Removing only one or a few lymph nodes lowers the risk of side effects from the surgery, such as arm swelling that is also known as lymphedema.
- Axillary lymph node dissection (ALND) is a procedure in which the surgeon removes many (usually less than 20) underarm lymph nodes. ALND is not done as often as it was in the past, but it might still be the best way to look at the lymph nodes in some situations[9].
Chemotherapy[edit | edit source]
Chemotherapy is used to destroy the remaining cancer cells that may be left within the body. This form of treatment is applied to the whole body through the bloodstream. Chemotherapy can be used with all stages of breast cancer but is especially recommended for those patients in which cancer has spread.
See Chemotherapy Side Effects and Syndromes
Radiation Therapy[edit | edit source]
Radiation therapy is typically used for early stages (can be used in all stages) of breast cancer following a lumpectomy. This form of treatment targets a more specific area unlike chemotherapy. Radiation therapy may also be used following chemotherapy.
- Almost half of cancer patients will use radiotherapy over the course of their cancer treatment.
See Radiation Side Effects and Syndromes
Hormonal Therapy[edit | edit source]
- Some types of breast cancer are affected by hormones, like estrogen and progesterone. The breast cancer cells have receptors (proteins) that attach to estrogen and progesterone, which helps them grow. Treatments that stop these hormones from attaching to these receptors are called hormone or endocrine therapy.
- Hormone therapy can reach cancer cells almost anywhere in the body and not just in the breast. It's recommended for women with tumors that are hormone receptor-positive. It does not help women whose tumors don't have hormone receptors.[9]
Medications[edit | edit source]
Medications for the treatment of breast cancer most often include chemotherapy drugs and hormone replacement drugs.
Chemotherapy medications are many times used in combinations of two or three at a time.
- Two common groups include anthracyclines and taxanes.
- Anthracyclines such as, Epirubicin and Doxorubicin, are similar to antibiotics that destroy the cancer cells’ genetic material.
- Taxanes such as Paclitaxel and Docetaxel, on the other hand, interfere with how the division of the cancer cells.[30]
- Paclitaxel and Docetaxel are both categorized as plant alkaloid anticancer drugs. Each are given intravenously and used mostly to treat solid tumors involving breast and ovarian cancers.
- Tamoxifen stop the growth, spread, or recurrence of ER-positive tumors by preventing estrogen from reaching the tumors. Tamoxifen is a mixed estrogen antagonist and agonist that blocks the estrogen activation in the breast and decreases growth factors in the breast tissue. Tamoxifen is the most common drug used for premenopausal women to help prevent the recurrence of breast cancer and another drug,
- Toremifene is the newer estrogen receptor antagonist that is being used in cases of advanced breast cancer.[26][27]
Physical Therapy Management[edit | edit source]
see also Oncology Examination
Post breast cancer treatment, women may experience any of the following impairments:
- Decreased strength of the upper extremity
- Decreased shoulder mobility
- Scar tightness (breast and/or axilla)
- Upper extremity ache
- Lymphedema of the upper extremity
- Neuropathic pain
- Musculoskeletal pain (breast, axilla, and/or neck-shoulder)
- Chronic pain
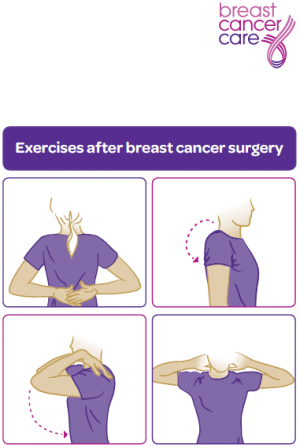
Interventions Post Surgery[edit | edit source]
A physiotherapists treatment plan should include:
- Motion exercises to improve tissue extensibility and facilitate normal movement patterns.
- Myofascial release for enhancing mobility and enhancing tissue extensibility. [31] [32] [33] [34]
Several forms of manual therapy may also assist:
- Joint mobilization techniques
- Soft tissue release techniques
- Neurodynamic techniques
- Muscle groups that should be targeted include the rotator cuff, serratus anterior, trapezius, rhomboids, biceps, and pectoralis muscles.[35] Exercises can begin with an elastic bands and be performed 2x/week for 2 sets of 10-15 repetitions.[36]
A structured Prevention of Shoulder Problems Trial (PROSPER) exercise programme introduced at one week post-operatively improved upper limb function, postoperative pain, arm symptoms, and physical quality of life at 12 months, compared with usual care alone, in women at high risk of upper limb disability after undergone non-reconstructive surgery.[37]
Mobility exercises[edit | edit source]
- Two common complications are restricted arm motion and lymphedema.
- Early rehabilitation is implemented to promote functional movement to the patient’s previous level of activity.
- Arm mobilisations are implemented first or second-day post-op.
- Mobilisations are performed using joint rotations to tolerance but abduction and flexion are limited to 40°.
- At day 4 post-op flexion and abduction are gradually increased to 45°, this can be increased furthermore by 10-15° per day dependent on the patient’s pain tolerance.
- The technique performed by holding the patients arm in 45° flexion or abduction until the drains are removed.
Surface electromyography study showed alterations in the amplitude of muscle activity and the onset in each of the selected shoulder movements among the women after breast cancer treatment, suggesting a need to develop a selective therapeutic exercise program optimizing the shoulder neuromuscular activity in women post breast cancer treatment[38].
Secondary lymphedema is a common occurrence in the breast cancer population following surgery and has a long term negative effect on patient quality of life. This can be treated with Complete Decongestive Therapy.
Physical Activity[edit | edit source]
- Exercise is increasingly being implemented as a therapeutic tool in patients with breast cancer [39]. In recent times it has become clear that exercise has a central role to play in controlling and preventing chronic illness.
- Statistically breast cancer survivors have a very low compliant rate, despite the renowned benefits of exercise.
- There is substantial evidence to support the benefits of exercise in breast cancer both during and after chemotherapy.
- Research has shown that physical activity and exercise is effective in improving quality of Life, cardiorespiratory fitness, physical functioning in breast cancer patients and survivors [40].
- Physical exercise has shown to be a suitable adjunct therapy to battle long term chronic conditions and has been successful in reducing mortality and improving overall quality of life.
Precautions
When performing exercise for post surgical populations the SEWS chart should be monitored regularly for early warning signs. If the patient is feeling fatigued or anaemic exercise should be delayed.
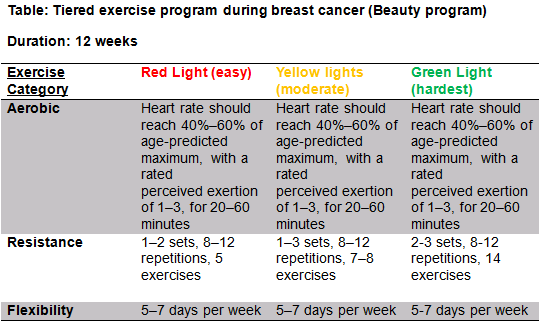
BEAUTY (see table R)[edit | edit source]
- The BEAUTY program aims to counteract key concerns associated with breast cancer patients such as fatigue, reduced QoL, social anxiety and physical conditioning.[41]
- Considering there is huge physiological benefits as well major psychological benefits it is important that the physiotherapist promotes the benefits of exercise immediately post-surgery and ensures that the exercise program is assessable at home or in the community and is specific to the individual.
- All exercise programs should be designed with F.I.T.T principles during and after breast cancer.
FITT Guidelines[edit | edit source]
Exercise compliance post cancer is very low [42], numerous factors for this such as lack of availability of services, travel issues, cost and personal reasons and fatigue are often reasons for this. Physiotherapist should be aware of the barriers to exercise compliance in this specific population (See #Barriers ).
FITT Principle After Breast Cancer[edit | edit source]
- Warm up: 5-10 minutes to raise heart rate
- Aerobic Exercise: Frequency:
- 3 x 5 times per week **Intensity: 50-70% of max. heart rate
- Type: walking cycling aerobic activity
- Time: 30 minutes maintaining as a long term routine
- Resistance Training: Frequency:
- 2/3 times a week
- Intensity: 12/15 reps of 60 % of 1RM
- Type: Supervised resistance program of major muscle groups
- Time: 6 weeks
Aerobic exercise, such as walking, cycling, or swimming, has been shown to decrease cancer-related fatigue,[43][44][45] improve quality of life,[46][47] reduce cognitive impairments associated with various cancer therapies,[48] improve cardiovascular outcomes,[49] and improve sleep dysfunction.[50] Research suggests that treadmill exercises provide cardioprotective effects on the Doxorubicin-induced cardiotoxicity.[51] Another study reported the positive effects of a 7- week pedometer exercise program on fatigue, quality of life, skeletal mass and functional capacity of the patients with breast cancer receiving chemotherapy.[52] It can be concluded that a supervised exercise program combining aerobic and resistance training has great benefits on fitness, bone health and quality of life especially in overweight or obese breast cancer survivors.[53]
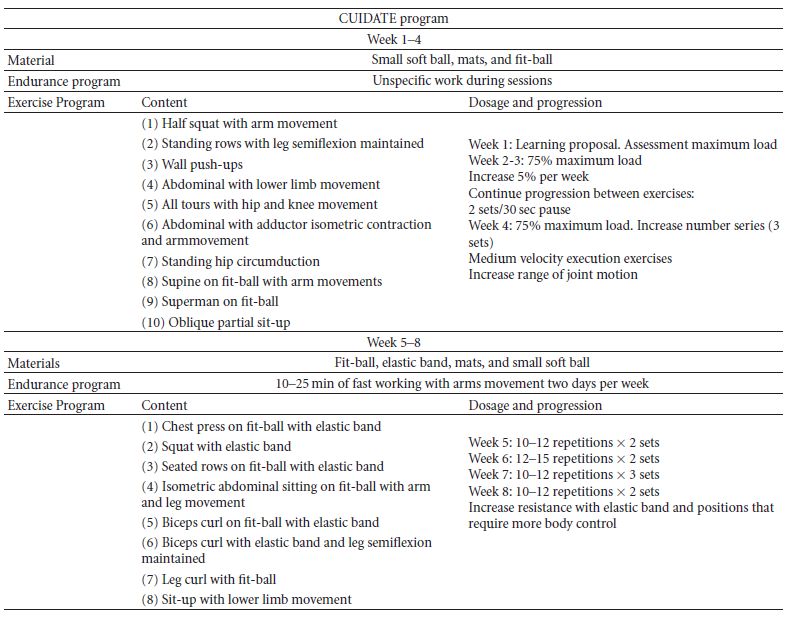
Below is a 8-week multimodal physiotherapy program (aerobic exercises, core stability exercises, and some recovery with stretching and myofascial release techniques).[54][50].
Physiotherapy Long-term Management[edit | edit source]
The role of a physiotherapist is to promote a healthy life style including physical activity and proper nutrition.
Exercise[edit | edit source]
- Continuation of exercise can continue to foster motivation in patients, provide a support group for patients, enable social and psychological wellbeing.
- It can improve patients quality of life.
- It allows patients to have some control over their lives, stability and routine.
- It allows them to regain themselves and return to being active in a community [55].
Education[edit | edit source]
- Education of the patient is a key component of the physiotherapists role.
- Promotion of physical activity, independence and self-management as greatly important for successful rehabilitation outcomes.
- In reference to the biopsychosocial model of health, physiotherapists should address more than just the patient’s physical problems. All patient needs and concerns need to be treated or referred to appropriate professionals.
Life After Cancer[edit | edit source]
Life after breast cancer treatment means returning to some familiar things and also making some new choices.
- The end of treatment does not mark the end of the journey with breast cancer.
- Two of the more frustrating and troubling side effects women face after treatment are fatigue resulting from chemotherapy and/or the accumulated effects of other treatments, and a phenomenon some women have dubbed "chemobrain" -- mental changes such as memory deficits and the inability to focus.
The physiotherapist can assist the patient with her plans to return to work by carrying out assessments on the physical capabilities of the patient in relation to the work place.
- A work place assessment will also benefit the achievement of this goal.
- Following the workplace assessment, an adjustment of the duties can be recommended to the patient and the employer.
- The knowledge of anatomy, kinesiology and ergonomics, together with the agreed work place adjustments, will allow the physiotherapist to focus on the treatment of the disease and prevent injuries when the patient returns to work.
Outcome Measures[edit | edit source]
| Lymphodema
LYMQOL is a validated lymphoedema specific outcome measure for QOL [56]. It consists of 24 questions covering 4 domains (symptoms, body image, mood and function. It is measured by a likert scale from 1-4 Cancer Related Fatigure BFI (brief fatigue inventory). The BFI measures the severity and impact of fatigue in a 24 hr duration. 9 items 0-10 numeric scale. [57] The functional assessment of cancer therapy (FACT-F) FACT-F measures physical fatigue and its consequences over a 7 day period. It is a 13-item uni-dimensional scale assessed on a 5-point scale of 0–4. [58] Shoulder Function Disabilities of the Arms, Shoulder, and Hand (DASH). [59] Psychometric Outcome Measure Hospital Anxiety and Depression Scale (HADS). [60] Quality of Life European Organisation for Research & Treatment of Cancer Breast Cancer – Quality of Life Questionnaire-Core 36 (EORTC QLQ-C36) Developed in 1987 by Aaronson et al. |
Resources[edit | edit source]
You can visit some of the websites listed below for more resources.
Download a PDF on Oncology and Breast Cancer
References[edit | edit source]
- ↑ 1.0 1.1 1.2 Radiopedia Breast cancer Available from:https://radiopaedia.org/articles/breast-cancer-summary?lang=gb (last accessed 22.8.2020)
- ↑ 2.0 2.1 Islami F, Guerra CE, Minihan A, Yabroff KR, Fedewa SA, Sloan K, Wiedt TL, Thomson B, Siegel RL, Nargis N, Winn RA. American Cancer Society's report on the status of cancer disparities in the United States, 2021. CA: a cancer journal for clinicians. 2022 Mar;72(2):112-43.
- ↑ Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: a cancer journal for clinicians. 2018 Nov;68(6):394-424.
- ↑ 4.0 4.1 Czajka ML, Pfeifer C. Breast Cancer Surgery. April 2020 Available from:https://www.ncbi.nlm.nih.gov/books/NBK553076/ (last accessed 22.8.2020)
- ↑ Doyle C, Kushi LH, Byers T, Courneya KS, Demark‐Wahnefried W, Grant B, McTiernan A, Rock CL, Thompson C, Gansler T, Andrews KS. Nutrition and physical activity during and after cancer treatment: an American Cancer Society guide for informed choices. CA: a cancer journal for clinicians. 2006 Nov;56(6):323-53.
- ↑ Rangel J, Tomás MT, Fernandes B. Physical activity and physiotherapy: perception of women breast cancer survivors. Breast Cancer. 2019 May;26(3):333-8.
- ↑ Medical news Breast cancer Available from:https://www.news-medical.net/health/Breast-Cancer-Pathophysiology.aspx (last accessed 23.8.2020)
- ↑ 8.0 8.1 8.2 Radiopedia Breast neoplasms Available from:https://radiopaedia.org/articles/breast-neoplasms (last accessed 22.8.2020)
- ↑ 9.0 9.1 9.2 ACS Breast cancer Available from:https://www.cancer.org/cancer/breast-cancer/treatment/surgery-for-breast-cancer.html (last accessed 23.8.2020)
- ↑ 10.0 10.1 Merkle CJ, Loescher LJ. Biology of cancer. Cancer nursing, principles and practice, 6th edn. Jones and Bartlett, Boston. 2005:3-26.
- ↑ Canadian Cancer Society. Breast Cancer. Available from: https://www.cancer.ca/en/cancer-information/cancer-type/breast/breast-cancer/?region=on [Accessed 2020 June 23].
- ↑ Spittler CA. Breast reconstruction using tissue expanders: assessing patients' needs utilizing a holistic approach. Plastic surgical nursing. 2008 Jan 1;28(1):27-32.
- ↑ NCI Staging Available from:https://seer.cancer.gov/tools/ssm/ (last accessed 23.8.2020)
- ↑ Canadian Cancer Society. Metastatic cancer. Available from: https://www.cancer.ca/en/cancer-information/cancer-type/metastatic-cancer/metastatic-cancer/?region=on [Accessed 2020 June 23].
- ↑ Breastcancer.org. Metastatic Breast Cancer Symptoms and Diagnosis. Available from: https://www.breastcancer.org/symptoms/types/recur_metast/metastic [Accessed 2020 June 23].
- ↑ Coleman MP, Quaresma M, Berrino F, Lutz JM, De Angelis R, Capocaccia R, Baili P, Rachet B, Gatta G, Hakulinen T, Micheli A. Cancer survival in five continents: a worldwide population-based study (CONCORD). The lancet oncology. 2008 Aug 1;9(8):730-56.
- ↑ Gondos A, Chokunonga E, Brenner H, Parkin DM, Sankila R, Borok MZ, Chirenje ZM, Nyakabau AM, Bassett MT. Cancer survival in a southern African urban population. International Journal of Cancer. 2004 Dec 10;112(5):860-4.
- ↑ Yu XQ, O'Connell DL, Forman D. Comparison of cancer survival in UK and Australia: rates are higher in Australia for three major sites. British journal of cancer. 2004 Nov;91(9):1663-5.
- ↑ Gorey KM, Holowaty EJ, Fehringer G, Laukkanen E, Richter NL, Meyer CM. An international comparison of cancer survival: relatively poor areas of Toronto, Ontario and three US metropolitan areas. Journal of Public Health. 2000 Sep 1;22(3):343-8.
- ↑ Petrek JA, Senie RT, Peters M, Rosen PP. Lymphedema in a cohort of breast carcinoma survivors 20 years after diagnosis. Cancer. 2001 Sep 15;92(6):1368-77.
- ↑ Ezzo J, Manheimer E, McNeely ML, Howell DM, Weiss R, Johansson KI, Bao T, Bily L, Tuppo CM, Williams AF, Karadibak D. Manual lymphatic drainage for lymphedema following breast cancer treatment. Cochrane database of systematic reviews. 2015(5).
- ↑ Ozmen V, Ilgun S, Ozden BC, Ozturk A, Aktepe F, Agacayak F, Elbuken F, Alco G, Ordu C, Iyigun ZE, Emre H. Comparison of breast cancer patients who underwent partial mastectomy (PM) with mini latissimus dorsi flap (MLDF) and subcutaneous mastectomy with implant (M+ I) regarding quality of life (QOL), cosmetic outcome and survival rates. World Journal of Surgical Oncology. 2020 Dec;18(1):1-2.
- ↑ Winchester DJ, Chang HR, Graves TA, Bland KI, Winchester DP. A comparative analysis of lobular and ductal carcinoma of the breast: presentation, treatment, and outcomes. Journal of the American College of Surgeons. 1998 Apr 1;186(4):416-22.
- ↑ Garduño-Ramón MA, Vega-Mancilla SG, Morales-Henández LA, Osornio-Rios RA. Supportive noninvasive tool for the diagnosis of breast cancer using a thermographic camera as sensor. Sensors. 2017 Mar;17(3):497.
- ↑ 25.0 25.1 What You Need to Know About Breast Cancer. National Cancer Institute. http://www.cancer.gov/cancertopics/wyntk/breast/allpages. (accessed 21 February 2010)
- ↑ 26.0 26.1 26.2 26.3 Goodman CC, Fuller KS. Pathology: Implications for the Physical Therapist, 3rd Ed. St. Louis, MO: Saunders Elsevier, 2009, pp 1015-1036. (Loe 1b)
- ↑ 27.0 27.1 Panus PC, Katzung B, Jobst EE, Tinseley SL, Masters SB, Trevor AJ. Pharmacology for the Physical Therapist. Cancer Chemotherapy. New York: McGraw-Hill Companies, Inc., 2009. p460-477.
- ↑ Goodman C, Snyder T. Differential Diagnosis for Physical Therapists: Screening for Referral. St. Louis, Missouri: Saunders Elsevier, 2007. p784-793.
- ↑ Czajka ML, Pfeifer C. Breast Cancer Surgery.April 2020 Available from:https://www.ncbi.nlm.nih.gov/books/NBK553076/ (last accessed 23.8.2020)
- ↑ BreastCancer.org. http://www.breastcancer.org/ (accessed 21 February 2010).
- ↑ McAnaw MB, Harris KW. The Role of Physical Therapy in the Rehabilitation of Patients with Mastectomy and Breast Reconstruction. Breast Disease 2002 12;16(1):163-174.
- ↑ Levangie PK, Drouin J. Magnitude of late effects of breast cancer treatments on shoulder function: a systematic review. Breast Cancer Res Treat 2009;116(1):1-15.
- ↑ EBAUGH, D., SPINELLI, B. AND SCHMITZ, K.H., 2011. “Shoulder impairments and their association with symptomatic rotator cuff disease in breast cancer survivors”, Medical Hypotheses. Vol. 77, pp. 481–487.
- ↑ Pacurar R, Miclaus C, Miclaus M. Morbidity associated with breast cancer therapy and the place of physiotherapy in its management. Timisoara Physical Education & Rehabilitation Journal 2011 05;3(6):46-54.
- ↑ Shamley D, Lascurain‐Aguirrebeña I, Oskrochi R. Clinical anatomy of the shoulder after treatment for breast cancer. Clinical Anatomy. 2014 Apr;27(3):467-77.
- ↑ Giacalone A, Alessandria P, Ruberti E. The physiotherapy intervention for shoulder pain in patients treated for breast cancer: Systematic review. Cureus. 2019 Dec;11(12).
- ↑ Bruce J, Mazuquin B, Canaway A, Hossain A, Williamson E, Mistry P, Lall R, Petrou S, Lamb SE, Rees S, Padfield E. Exercise versus usual care after non-reconstructive breast cancer surgery (UK PROSPER): multicentre randomised controlled trial and economic evaluation. bmj. 2021 Nov 11;375.
- ↑ Prieto-Gómez V, Navarro-Brazález B, Sánchez-Méndez Ó, de-la-Villa P, Sánchez-Sánchez B, Torres-Lacomba M. Electromyographic Analysis of Shoulder Neuromuscular Activity in Women Following Breast Cancer Treatment: A Cross-Sectional Descriptive Study. Journal of Clinical Medicine. 2020 Jun;9(6):1804.
- ↑ Courneya KS. Exercise in cancer survivors: an overview of research. Medicine & Science in Sports & Exercise 2003 11;35(11):1846-1852.
- ↑ PANEL E. American College of Sports Medicine roundtable on exercise guidelines for cancer survivors. 2010.
- ↑ Leach H, Danyluk J, Culos–Reed S. Design and implementation of a community-based exercise program for breast cancer patients. Current Oncology 2014;21(5):267.
- ↑ Miedema B, Easley J. Barriers to rehabilitative care for young breast cancer survivors: a qualitative understanding. Supportive Care in Cancer 2012;20(6):1193-1201.
- ↑ Cantarero-Villanueva I, Fernández-Lao C, Cuesta-Vargas AI, Del Moral-Avila R, Fernández-de-las-Peñas C, Arroyo-Morales M. The effectiveness of a deep water aquatic exercise program in cancer-related fatigue in breast cancer survivors: a randomized controlled trial. Archives of physical medicine and rehabilitation. 2013 Feb 1;94(2):221-30.
- ↑ Carter SJ, Hunter GR, McAuley E, Courneya KS, Anton PM, Rogers LQ. Lower rate-pressure product during submaximal walking: a link to fatigue improvement following a physical activity intervention among breast cancer survivors. Journal of Cancer Survivorship. 2016 Oct 1;10(5):927-34.
- ↑ Vardar Yağlı N, Şener G, Arıkan H, Sağlam M, İnal İnce D, Savcı S, Çalık Kutukcu E, Altundağ K, Kaya EB, Kutluk T, Özışık Y. Do yoga and aerobic exercise training have impact on functional capacity, fatigue, peripheral muscle strength, and quality of life in breast cancer survivors?. Integrative cancer therapies. 2015 Mar;14(2):125-32.
- ↑ Burnham TR, Wilcox A. Effects of exercise on physiological and psychological variables in cancer survivors. Medicine and science in sports and exercise. 2002 Dec 1;34(12):1863-7.
- ↑ McNeely ML, Campbell KL, Rowe BH, Klassen TP, Mackey JR, Courneya KS. Effects of exercise on breast cancer patients and survivors: a systematic review and meta-analysis. Cmaj. 2006 Jul 4;175(1):34-41.
- ↑ Campbell KL, Kam JW, Neil‐Sztramko SE, Liu Ambrose T, Handy TC, Lim HJ, Hayden S, Hsu L, Kirkham AA, Gotay CC, McKenzie DC. Effect of aerobic exercise on cancer‐associated cognitive impairment: A proof‐of‐concept RCT. Psycho‐oncology. 2018 Jan;27(1):53-60.
- ↑ Zhang Y, Xu L, Zhang X, Yao Y, Sun Y, Qi L. Effects of different durations of aerobic exercise on the cardiovascular health in untrained women: a meta-analysis and meta-regression.
- ↑ 50.0 50.1 Roveda E, Vitale JA, Bruno E, Montaruli A, Pasanisi P, Villarini A, Gargano G, Galasso L, Berrino F, Caumo A, Carandente F. Protective effect of aerobic physical activity on sleep behavior in breast cancer survivors. Integrative cancer therapies. 2017 Mar;16(1):21-31.
- ↑ Yang HL, Hsieh PL, Hung CH, Cheng HC, Chou WC, Chu PM, Chang YC, Tsai KL. Early Moderate Intensity Aerobic Exercise Intervention Prevents Doxorubicin-Caused Cardiac Dysfunction Through Inhibition of Cardiac Fibrosis and Inflammation. Cancers. 2020 May;12(5):1102.
- ↑ Gandhi A, Samuel SR, Kumar KV, Saxena PU, Mithra P. Effect of a Pedometer-based Exercise Program on Cancer Related Fatigue and Quality of Life amongst Patients with Breast Cancer Receiving Chemotherapy. Asian Pacific Journal of Cancer Prevention. 2020 Jun 1;21(6):1813-8.
- ↑ Dieli-Conwright CM, Courneya KS, Demark-Wahnefried W, Sami N, Lee K, Sweeney FC, Stewart C, Buchanan TA, Spicer D, Tripathy D, Bernstein L. Aerobic and resistance exercise improves physical fitness, bone health, and quality of life in overweight and obese breast cancer survivors: a randomized controlled trial. Breast Cancer Research. 2018 Dec;20(1):1-0.
- ↑ Cantarero-Villanueva I, Fernández-Lao C, del Moral-Avila R, Fernández-de-las-Peñas C, Feriche-Fernández-Castanys MB, Arroyo-Morales M. Effectiveness of core stability exercises and recovery myofascial release massage on fatigue in breast cancer survivors: a randomized controlled clinical trial. Evidence-Based Complementary and Alternative Medicine. 2012 Jan 1;2012.
- ↑ Unruh, A. and Elvin, N. (2004). In the eye of the dragon: Women's experience of breast caner and the occupation of dragon boat racing. R EVUE CANADIENNE D ’ ERGOTHÉRAPIE, 71(3), pp.138-149.
- ↑ Keeley V, Crooks S, Locke J, Veigas D, Riches K, Hilliam R. A quality of life measure for limb lymphoedema (LYMQOL). J LYMPHOEDEMA 2010 04;5(1):26-37.
- ↑ Mendoza TR(1), Wang XS(1), Cleeland, C.S. ( 1,4 ), Morrissey M(1), Johnson BA(1), Wendt JK(1), et al. The rapid assessment of fatigue severity in cancer patients: Use of the brief fatigue inventory. Cancer 1999 / 01 / 01 /;85(5):1186-1196.
- ↑ Yellen, S.B. ( 1,3 ), Cella DF(1), Webster K(1), Blendowski C(1), Kaplan E(2). Measuring fatigue and other anemia-related symptoms with the Functional Assessment of Cancer Therapy (FACT) measurement system. J Pain Symptom Manage 1997 / 02 / 01 /;13(2):63-74.
- ↑ Hudak, P.L. ( 1,2 ), Amadio, P.C. ( 1,3 ), Bombardier, C. ( 2,4 ). Development of an upper extremity outcome measure: The DASH (disabilities of the arm, shoulder, and head). Am J Ind Med 1996 / 01 / 01 /;29(6):602-608
- ↑ Zigmond AS, Snaith RP. The Hospital Anxiety and Depression Scale. Acta Psychiatr Scand 1983 06;67(6):361.
Top Contributors - Adam El-Sayed, Kim Jackson, Lucinda hampton, Vidya Acharya, Admin, Manisha Shrestha, 127.0.0.1, Chee Wee Tan, Ilona Malkauskaite and Candace Goh References